3 Phoglyceric Acid Pka , D-(−)-3-Phosphoglyceric Acid (sodium salt) (CAS 80731-10-8)
Di: Luke
Conversely, for the titration of a weak . 3-Hydroxypropionic acid is used in the industrial production of various .
pH, pKa, and the Henderson-Hasselbalch Equation
Each reaction is catalyzed by a specific enzyme.pKa Data Compiled by R.Calculations and expressions involving Ka and p Ka were covered in detail in your first-year general chemistry course. Community Flexikon Shop News Jobs CME Flexa.two molecules of phosphoglycerate (PGA), a three-carbon acid.In 3-chlorobutanoic acid, the pKa is 4. Sử dụng các giá trị pKa, người ta có thể thấy axit lactic là một axit mạnh hơn axit axetic.What is of interest to us here is the relationship between pK a and acidity. Calculate the pH of a solution with a concentration of 0. Tables of pK a values usually show the . Williams pKa Values INDEX Inorganic 2 Phenazine 24 Phosphates 3 Pyridine 25 Carboxylic acids 4, 8 Pyrazine 26 Aliphatic 4, 8Consider, for example, acetic acid, which in aqueous solution has a pKa of about 4.1–3 By now, accurate and precise determination of pH seems to have few secrets left.0, whereas in 4-chlorobutanoic acid, with the fluorine all the way at the end of the chain, six bonds away from the acidic position, the pKa is 4. Six revolutions of the cycle means that 6 CO 2 .0 × 10 −7: 6. Por exemplo, o pKa do ácido acético é 4,8, enquanto o pKa do ácido lático é 3,8. Two molecules of 3-Phosphoglyceric acid (3PGA) are produced when CO 2 interactes with the sugar RuBP in the first reaction of the Calvin cycle in .7 × 10 −5: 4.3-Phosphoglyceric acid.If there is a side chain pKa involved, you take the 2 pKa values that are closest together, add them, and then divide by 2. Use the Henderson-Hasselbalch equation: pH = pKa + log 10.1, we see that the pKa of HSO − 4 is 1. Sulfuric acid –1. To simplify the numbers, -log Ka is used instead of the K a getting rid of the exponent. Perchloric acid –7.com3-Phosphoglyceric acid – GSUhyperphysics. For simplicity, if Compound A has a pKa of 4 and is in a . Consider, for example, the HSO − 4 / SO2 − 4 conjugate acid–base pair.Ausser Betrieb Der Service www.1, compared to HCN which has a pKa value of 9. pK a là logarit cơ số 10 âm của hằng số phân ly axit (K a) của một dung dịch.4: pKa Table – Chemistry LibreTextschem. pKa = -log 10 K a Quanto menor o valor de pKa , mais forte é o ácido .In the previous post, we talked about the acid strength and its quantitative description by pKa.orgEmpfohlen auf der Grundlage der beliebten • Feedback
3-Phosphoglyceric acid
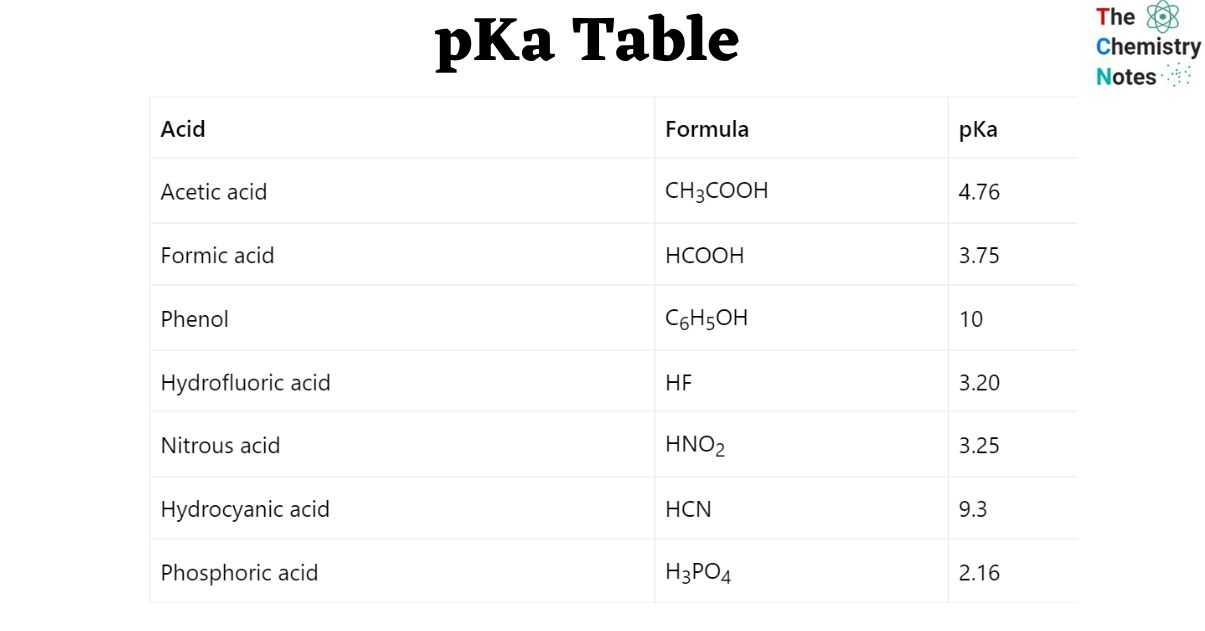
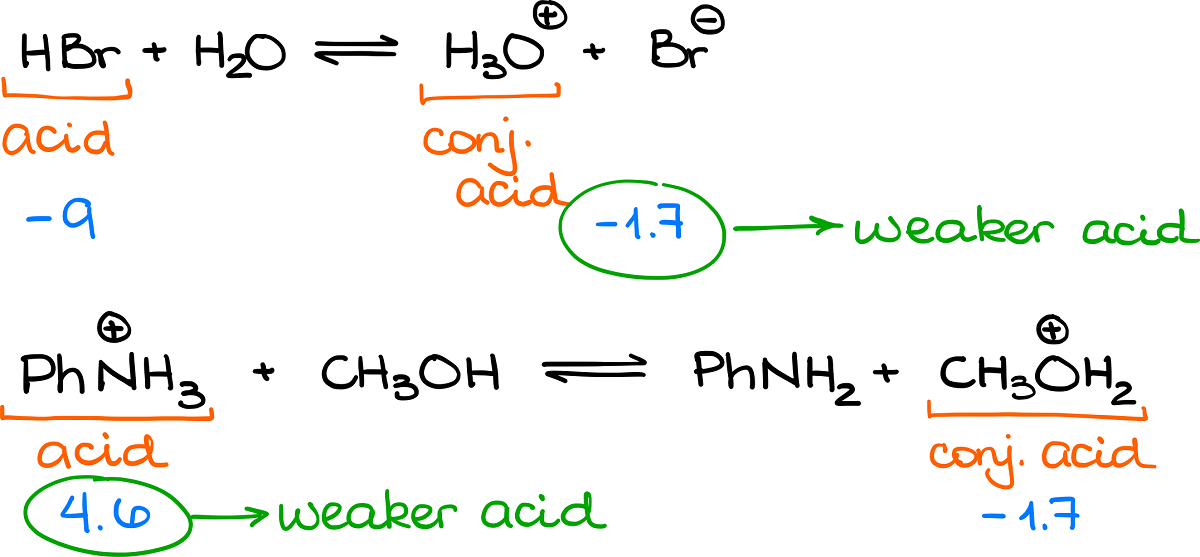
comPurpose of the conversion of 3-PGA to G3P in the calvin cycle?biology. However, in the gas phase the . The lower the pKa of a Bronsted acid, the more easily it gives up its proton. Notice that the methoxy group increases the . S3 and S4 in the Supplementary Information).For example, And a weaker . The stronger the acid (i.pKa Table – Scientific ToolKitscitk.Definitions of the acid dissociation constant and pKa are given below the figures, together with the definition of some classes of organic acids .7 for -NH 3(+). Sie besitzt jeweils zwei regulatorische und zwei katalytische .The strength of an acid is determined by its pKa value; the lower the pKa, the stronger the acid. The Henderson-Hasselbalch equation relates pKa and pH.
Proteinkinase A
pK a é o logaritmo negativo da base 10 da constante de dissociação ácida (Ka ) de uma solução .uk3-phosphoglycerate | chemical compound | Britannicabritannica.Like any other conjugate acid–base pair, the strengths of the conjugate acids and bases are related by pKa + pKb = pKw. To determine how much stronger one acid is compared to another, we need to look at the difference in pKa values. pKa = −logKa = log( 1 Ka) p K a = − l o g K a = l o g ( 1 K a) shows that such relationship is inverse.3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P).gov3-Phosphoglyceric Acid – an overview | ScienceDirect Topicssciencedirect.0, so an indicator such as phenolphthalein or thymol blue, with pKin > 7.6, the results are usually fatal.

5 × 10 −4: 3.46: Dichloroacetic acid: CHCl 2 CO 2 H: 4. For our two compounds, we have.
pKa Values INDEX

Consider the acidity of 4-methoxyphenol, compared to phenol. An isolectric pH is the pH at which a given amino acid has a net zero charge. The pKa table shows how greatly the acidity varied for different functional groups. Out of service The service www. H 3 O + Hydronium –1.The pKa is the pH value at which a chemical species will accept or donate a proton.0, should be used. Molecular Formula.By this we mean that the equilibrium position for the proton-transfer reaction lies more on the side of ROH as R is changed from primary to secondary to tertiary; therefore, tert-butyl alcohol is considered less acidic than ethanol: ROH + OH− ⇌ RO− + HOH (13.

Vinylagous Acids. 2R-hydroxy-3-(phosphonooxy)-propanoic acid, disodium salt. NEU: Log dich ein, um Artikel in persönlichen . The centennial of the concept and of the quantitative measurement of pH was celebrated not long ago. Use a resonance argument to explain why picric acid has such a low pKa.In short, the stronger the acid, the smaller the pKa value and strong acids have weak conjugate bases.42 54 C6H5CH2- 2. Make a structural argument to account for its strength.3-Phosphoglyceric acid ( 3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate ( GP or G3P ).Neither good correlation is observed between the atomic charge of boron in the neutral nor ionic form with the pK a of meta-substituted phenylboronic acid (cf. These ions are moderately stable in water but reassociate readily to form the starting product. pKa = – logKa.Upon distillation, it dehydrates to form acrylic acid, and is occasionally called hydracrylic acid.Definição de pKa.2 × 10 −7: 6.If the pH of human blood, for instance, gets outside the range 7.4 × 10 −4: 3.
3-Phosphoglyceric acid
These plants are called “C 3 ” due to the three-carbon compound (3-phosphoglyceric acid, or 3-PGA) produced by the Calvin–Benson cycle. It may be a larger, positive number, such as 30 or 50. Die Proteinkinase A, kurz PKA, ist ein Enzym aus der Gruppe der Serin/Threonin-Kinasen. The phenol derivative picric acid has a pKa of 0.The pKa measures how tightly a proton is held by a Bronsted acid. Ví dụ, pKa của axit axetic là 4,8, trong khi pKa của axit lactic là 3,8. Each molecule can contain a side chain or R group, e. It is very soluble in water, soluble in ethanol and diethyl ether. the higher its acidity constant K a ), the lower its pK a value, and viceversa. This glycerate is a .3-phosphoglyceric acid (CHEBI:17050) – European . C3H5O7P • 2Na.
55 57 2-phosphoglyceric acid 1.Technical Information. In solution, amino acids have various states to which they are charged (protonated). Chemical Formula. Hence the pKb of SO2 − 4 is 14.Empfohlen auf der Grundlage der beliebten • Feedback
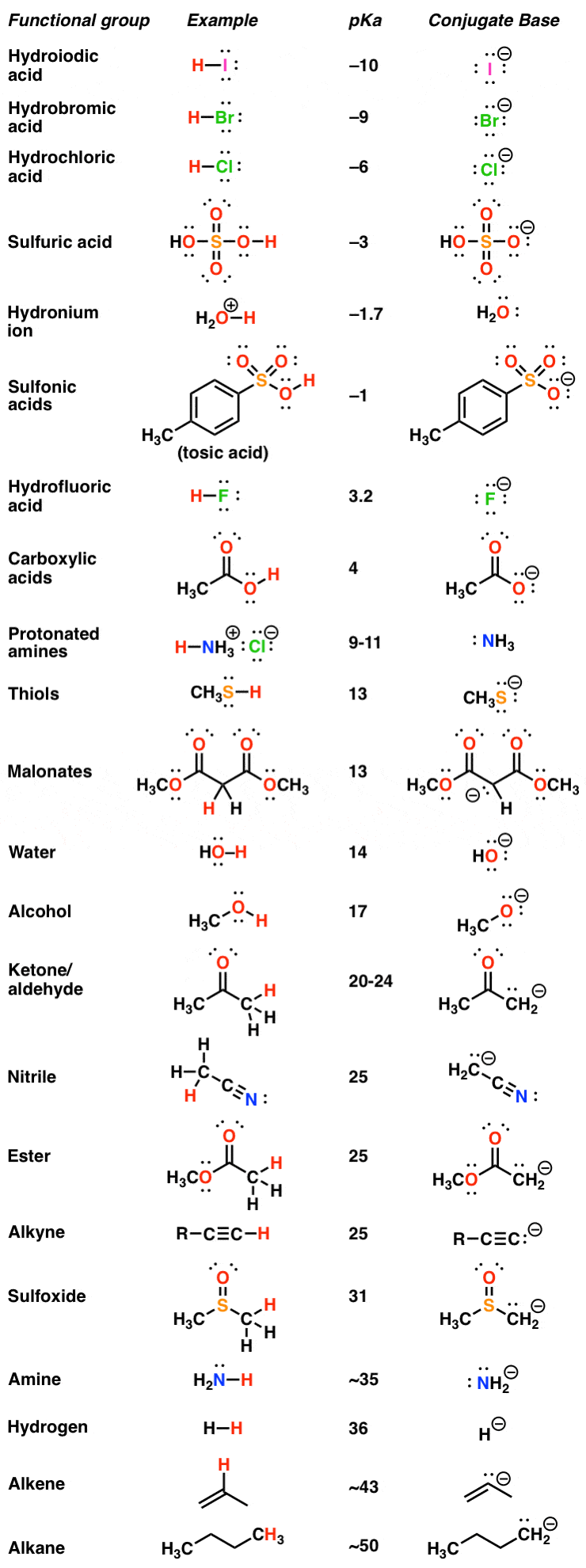
Bordwell pKa Table
This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin .
3-Phosphoglyceric Acid
Nitric acid +1. pKa1 = – log10-16 = 16 < pKa2 = – log10-38 = 38. There probably exist a number of . It is a weak acid, which dissociates only slightly to form H+ (in water the hydronium ion, H 3 O +, is formed) and acetate (Ac-).Abbreviations: PEP, phospho(enol)pyruvic acid; OAA, oxaloacetic acid; RuDP, ribulose 1,5-diphosphate; PGA, 3-phosphoglyceric acid. Negative pH values are only for academic exercises. For the titration of a weak acid, however, the pH at the equivalence point is greater than 7. pKa = -log 10 K a Giá trị pK a càng thấp thì axit càng mạnh. The related concept of the acid dissociation constant (pK a) as a substance property is recognized as being among the most commonly used .25, lower than that of trifluoroacetic acid.
Phosphoglyceric Acid
The pH of blood is controlled by the buffering action of several conjugate acid-base pairs.Mit Phosphoglycerinsäuren (kurz: PGS) werden zwei chemischen Verbindungen, 2-Phosphoglycerinsäure (auch Glycerinsäure-2-phosphat) und 3-Phosphoglycerinsäure . Note that acidity constant is also known as the acid .The alcohol is a stronger acid because its K a is higher. b) Nitric acid is a strong acid – it has a pK a of -1.In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pK in between about 4. Alanine is an example of standard amino acid containing methyl .Ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase, or simply RuBisCO, is an enzyme which catalyses the carboxylation of RuBP to produce two molecules of d-3 .It is an acidic viscous liquid with a pKa of 4. The isoelectric points range from 5.4 for -CO 2 H, and 8. You can see that hydroxide ion is a stronger base than ammonia (NH 3 ), because ammonium (NH 4+, pK a = 9. Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco; EC 4.For example, The question is how do we explain these differences? Well, remember, we said that the stronger the acid, the weaker its conjugate base. A pKa may be a small, negative number, such as -3 or -5. Your answer should involve the structure of nitrate, the conjugate base of . pKa values describe the point where the acid is 50% dissociated (i.comEmpfohlen auf der Grundlage der beliebten • Feedback
E5: Acid Dissociation Constants of Organics
3-phosphoglyceric acid 1. Starting from a fully protonated state, the pK a ’s of the acidic functions range from 1. Titration curves show the neutralization of these acids by added base, and the change in pH .We seldom say the pH is 0, and that is why you consider pH = 0 such an odd expression. The fluorine in these last two cases has relatively little effect.01 M acetic acid. To learn more about Calculation of pka, List of pKa values, Relationship between pKa and pH and FAQs of pKa, Visit BYJU’S3-Hydroxypropionic acid is a carboxylic acid, specifically a beta hydroxy acid.Example 1: The pKa of acetic acid is 4.Die Abkürzung PKA hat verschiedene Bedeutungen: Einloggen.40: Cyanic acid: HCNO: 3.39) catalyzes the addition of gaseous carbon dioxide to ribulose-1,5-bisphosphate (RuBP), . Nitro groups are very powerful electron-withdrawing groups. However, it is only an approximation and should not be used for concentrated solutions . When we compare these values with those of comparable alcohols, such as ethanol (pK a = 16) and 2-methyl-2-propanol (pK a = 19), it is clear that carboxylic acids are stronger acids by over ten powers of ten! Furthermore, electronegative substituents .30 is equivalent to a \ (\ce { [H+]}\) of 2. Given: pKa = 4.
Hydrochloric acid –3.Sulfuric acid is the strongest acid on our list with a pK a value of –10, so HSO 4- is the weakest conjugate base.76 and concentration of acetic acid = 0. The p K a is the quantitative indicator of the acid strength.pKa – The pKa value is the negative base -10 logarithm of the acid dissociation constant (Ka) of a solution. It is a weak acid, which dissociates only slightly to form H + (in water the hydronium ion, H 3 O +, is formed) and acetate (Ac-).1 NH3+(CH2)4)- 2.1) R O H + O H − ⇌ R O − + H O H. The most important of these is undoubtedly the H 2 CO 3 /HCO 3 – pair, but side chains of the amino acid histidine in the hemoglobin molecule also .3-phosphoglyceric acid | chemical compound | Britannicabritannica.
Using the concentrations directly conveys a .1×10 –3: 2-nitrobenzoic .Indeed, a good correlation can be seen between .Properties of Amino Acids (pKa, pKb, pKx, pl) The properties of α-amino acids are complex, yet simplistic in that every molecule of an amino acid involves two functional groups: carboxyl (-COOH) and amino (-NH2). Contributors; The pK a ’s of some typical carboxylic acids are listed in the following table.55 57 peroxymonophosphoric acid 4. The lower the pKa, the stronger the acid and the greater the ability to donate a proton in aqueous solution. The quantitative behavior of acids and bases in solution can be understood only if their pKa values are known.

The pKa of acetic acid in 80% .Englisch: 3-phosphoglyceric acid, 3-PGA, PGA Definition 3-Phosphoglycerat , kurz 3PG , ist ein Stoffwechselintermediat der Glykolyse und der Gluconeogenese .A similar circumstance is also obtained for meta- substituted phenylboronic acid.This behavior is general for simple (difunctional) amino acids. Phosphoric acid has a pKa value of 2.3-Phosphoglyceric acid | C3H7O7P | CID 439183 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, . a) Draw the Lewis structure of nitric acid, HNO 3.
pKa and Acid Strength
3-Phosphoglyceric acid | C3H7O7P | CID 439183 – PubChempubchem.Định nghĩa pKa.3) Bei gegebenen pH einer wässrigen Lösung ergibt sich das Verhältnis von (korrespondierender) Säure AH zu (korrespondierender) Base A gemäss = K a .

D-(−)-3-Phosphoglyceric Acid (sodium salt) (CAS 80731-10-8)
ch steht von 00:00 bis 23:59 Uhr nicht zu Verfuegung. Inductive effects fall off quickly with increasing distance from the acidic site. In the table below, pK a1 and pK a2 for water solutions at 25°C are given together with boiling and melting point, density and molecular weight , as well as number of carbon, hydrogen and oxygen atoms in each . Given the pKa values for phosphoric acid .1×10 –3: nitrilotriacetic acid (T = 20 o C) (pK a1: μ = 0.
Amino Acids
49: Citric acid: C 6 H 8 O 7: 7. Usando os valores de pKa, pode-se ver que o ácido lático é um . The higher the pKa of a Bronsted acid, the more tightly the proton is held, and the less . Around 95% of the total green plants .
- 24 Stunden Pflege Wie Lange : Kostenrechner häusliche 24 Stunden Pflege (Seniorenbetreuung)
- 3 Wochen Altes Baby Schnupfen – Schnupfen beim Kleinkind: Tipps und Tricks zur Besserung
- 25010030 Postbank | Postbank: Pfändungsstelle
- 3 Zimmer Wohnung Neutraubling – Wohnungen mieten in Neutraubling
- 3840 X 2160 Wallpaper Gto , Gta 5 3840×2160 Resolution Wallpapers 4K
- 360 Grad Kamera Theta | Ricoh THETA V 360 Grad Sphärenkamera metallic grau
- 2X Bachelor Oder Master | Zweitstudium: Voraussetzungen, Gebühren, BaföG
- 30 Jahre Altes Mann | Altersunterschied in der Beziehung: Wie groß darf er sein?
- 3 Liga Tv _ Deutschland
- 30 Jahrestag Deutsche Einheit : 08:17 Ex-NATO-Strategin: Für Putin sind wir sehr berechenbar
- 22 Juli Netflix , Watch 22 July
- 3 In 1 Rücklichtkombination : Wechselschaltung Licht ⚡ Wechselschaltung anschliessen
- 30 Jahre Verheiratet Glückwünsche