7.6 Heats Of Reactions – Heat (Enthalpy) of Reaction: Definition, Examples, & Formula
Di: Luke
88 × 105g ⋅ m2.explain how heats of hydrogenation (ΔH° hydrog) can be used to show that cis alkenes are less stable than their trans isomers, and discuss, briefly, the limitations of this approach.1 can be rewritten to: w = mad. The system is performing work by lifting the piston against the downward force exerted by the atmosphere (i. Because both components of each compound change . In essence, Hess’s law enables us to calculate the enthalpy change for the sum of a series of reactions without having . Explain the technique of calorimetry. For a chemical reaction (the system) carried out at constant pressure – with the only work done caused by expansion or contraction – the . We now introduce two concepts useful in describing heat flow and temperature change. It is also toxic and a significant air pollutant, particularly in cities.Figure \(\PageIndex{1}\) Energy Changes Accompanying the Thermite Reaction Because enthalpy is a state function, the overall enthalpy change for the reaction of 2 mol of Al(s) with 1 mol of Fe 2 O 3 (s) is −851.The heat released by a reaction carried exit at constant volume is identical to the change in internal energy (ΔU) rather than and enthalpy change (ΔH); ΔU is similar at ΔH by an expression ensure depends on the change in the number of moles of gas during this reaction. ΔH = Hfinal − Hinitial = qp.1 kJ, whether the reaction occurs in a single step (ΔH 4, shown on the left) or in three hypothetical steps (shown on the right) that involve the . ΔHrxn = −qcalorimeter = −5.1 the sum of parts (a) + (b) + (c).2) AC + BD → AD i n s o l u b l e + BC.The oxygen atoms produced in Equation 7.7: Thermochemical Equations.3: Heats of Reactions and Calorimetry – Chemistry LibreTexts / . The magnitude of ΔH for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure of any gases present, and the temperature at which the reaction is carried out. Presented below: 2HCl(aq) + Zn(s) → ZnCl 2(aq) + H 2(g) is an example of a single-replacement reaction.chemdictionary. You can usually find this information in . Because all forms of energy can be interconverted, energy in any . From Equation 5.If the reaction releases heat (question rxn 0) and its temperature increases. For example, a large fire produces more heat than a single match, even though the . Usually a coffee-cup calorimeter is used since it is simpler than a bomb calorimeter, but to measure the heat evolved in a combustion reaction, constant volume or bomb calorimetry is ideal.
03 g of KOH in water is accompanied by the release of 5. The change in enthalpy is an extensive property .Learn chemistry with CK-12 Foundation’s interactive FlexBook, featuring simulations, videos, and practice questions on various topics.
Hess’s law allows us to calculate ΔH values for reactions that are difficult to carry out directly by adding together the known \(ΔH\) values for individual steps that give the .To calculate the heat of reaction numerically, you’ll need the standard enthalpies of formation for all products and reactants.If the reaction is carried out in a closed system that is maintained at constant pressure by a movable piston, the piston will rise as nitrogen dioxide gas is formed (Figure \(\PageIndex{5}\)).8 m/s (approximately 60 mi/h) is.Cause this calcium operates at constant volume, the heats published is not precisely the same as the enhalpy replace for the reaction.1 is identical to Equation 9.

The standard heat of formation is defined as the amount of heat absorbed or evolved at 25° C (77° F ) and at one atmosphere pressure when one mole of a compound is formed from . The hydrogen atoms in HCl are replaced by Zn atoms, and . Learn more at http://www.Apply a stoichiometric conversion factor to convert between the amount of heat that is transferred during a chemical reaction and the molar quantity of a substance that . The subscript p is used here to emphasize that this equation is true only for a process that occurs at constant pressure. We also acknowledge previous National Science Foundation support under .The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.2) Ozone is responsible for the pungent smell we associate with lightning discharges and electric motors. Heat of Reaction from Heats of .If the reaction releases heat (q rxn 0) press him cold increases.The magnitude of \(ΔH\) for a reaction is proportional to the amounts of the substances that react. Calculate and interpret heat and related properties using typical . Because the temperature of the solution increased, the dissolution of KOH in water must be exothermic.1 can undergo a condensation reaction with O 2 molecules to form ozone: O(g) +O2 (g) → O3(g) (7. The difference in the enthalpy of a specific chemical reaction is obtained at a constant pressure. Enthalpy is a state function used to measure the heat transferred from a system to its surroundings or vice versa at constant pressure. H 2 O is produced in the gaseous phase due to the high temperatures that accompany combustion reactions.It was released by KOH dissolving in water. 7: The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction.3: Heats of Reactions and Calorimetry – .
Heat of Reaction
Because the volume of the system (the interior of the bomb) is fixed, the combustion reaction occurs under conditions in which the volume, but not the pressure, is constant. Convert the temperature of the surface of the sun (5800 K) and the boiling points of gold (3080 K) and liquid nitrogen (77.2: Heat Changes during Chemical Reactions.3: Heats of Reactions and Calorimetry – Chemistry LibreTexts – Chem lab report 6 full . A constant volume . Thus, for the formation of FeO (s), Fe(s) + 1 2O2(g) → FeO(s) ΔH = ΔHf = − 272kJ / mol.It typical devices called calorimeters, who measure the change to temperature when a chemist reaction . The heat of reaction (ΔH) ( Δ H) is unchanged by the presence of the catalyst. Because the force (F) that opposes the action is equal to the mass (m) of the object times its acceleration ( a ), Equation 7.5: Enthalpy of reactions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts., atmospheric pressure). Here we shall .docx – Heats of Reaction Abstract The purpose of this experiment is to get a better understanding of heat transfer between | .Combustion is a reaction in which a substance reacts with oxygen gas. We find the amount of PV .Standard Enthalpies of Formation. Heat of Reaction from Heats of Formation. C 2 H 5 OH ( l) + O 2 ( g ) →.The net reaction in part (d) in Equation 9. The activation energy of the uncatalyzed reaction is shown by Ea E a, while the catalyzed reaction is shown by E′a E a ′.
Chemical Reactivity Hazards
It uses devices called calorimeters, which assess the change in temperature when a chemo reaction .1}\] Heat capacity is determined by both .orgEmpfohlen auf der Grundlage der beliebten • Feedback The heat capacity (C) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (ΔT) of 1 degree Celsius (or equivalently, 1 kelvin) \[C=\dfrac{q}{ΔT} \label{7.The kinetic energy of an object is related to its mass m and velocity v: KE = 1 2mv2. This experiment tells us that dissolving 5. For example, the kinetic energy of a 1360 kg (approximately 3000 lb) automobile traveling at a velocity of 26. Only the change in enthalpy (ΔH) can be measured.orgEnthalpy Change Definition, Types Of Enthalpy Change .ΔH = ΔU + PΔV = qp + w − w = qp. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments.0 bewilligung and was authored, remixed, and/or curated by LibreTexts. From Equation 6.The subscript f is the clue that the reaction of interest is a formation reaction. By Hess’s law, the enthalpy change for part (d) is the sum of the enthalpy changes for parts (a), (b), and (c). Since it also contains hydrogen, H 2 O will be another product.

Enthalpy (Heat) of Reaction and Thermochemical Equations
A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound, generating a new element and a new compound as products. Since C 2 H 5 OH contains carbon, CO 2 will be one of the products.This unbalanced equation has the general form of an exchange reaction: AC + BD → ADinsoluble + BC (7. Note that now we are using kJ/mol as the unit because it is understood that the enthalpy change is for one mole of substance. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble.There is only one temperature for which the numerical value is the same on both the Fahrenheit and Celsius scales: −40°C = −40°F. Reversing, supposing and reaction absorbs heat ( q rxn > 0), then heat is transferred from the calorimeter to the system ( q calorimeter < 0) and the temperature of the calorimeter decreases.13 kJ of energy. KE = 1 2(1360kg)(26.When chemical reactions are not properly managed, they can have harmful, or even catastrophic consequences, such as toxic fumes, fires, and explosions. For a chemical reaction (the system) carried out at constant pressure – with the only work done caused by expansion or contraction – the enthalpy of reaction (also called the heat of reaction, Δ Hrxn) is equal to the heat exchanged with the surroundings ( qp ). Generally, it is determined as the .
Heat (Enthalpy) of Reaction: Definition, Examples, & Formula
3: Heats of Reactions and Calorimetry.The heat of reaction is the heat required to effect the reaction, or the heat produced by the reaction – some authors use one definition, others use the other.3: Heats by Reactions and Calorimetry is collective under a CC BY-NC-SA 4.36 K) to °C and °F.
Enthalpy (Heat) of Reaction and Thermochemical Equations
Heats of reaction measure enthalpy changes, ΔH°, whereas equilibrium constants .The heat of reaction, also known as enthalpy of reaction, is the enthalpy change of a chemical reaction occurring at a constant pressure.Hess’s Law – Chemistry LibreTextschem. Determine if a chemical process is exothermic or endothermic.Second, heats of reaction and equilibrium constants don’t measure exactly the same thing.comEmpfohlen auf der Grundlage der beliebten • Feedback A student is ill with a temperature of 103. Learning Objectives.1: One form of energy is mechanical work, the energy required to move an object of mass m a distance d when opposed by a force F, such as gravity.1, we see that. The dissimilarity amongst the heat flow measured at const volume and the .
CHEM 117 Lab 4 – Lab Report on Heat Reactions – Studocustudocu. Note, too, by definition, that the enthalpy .
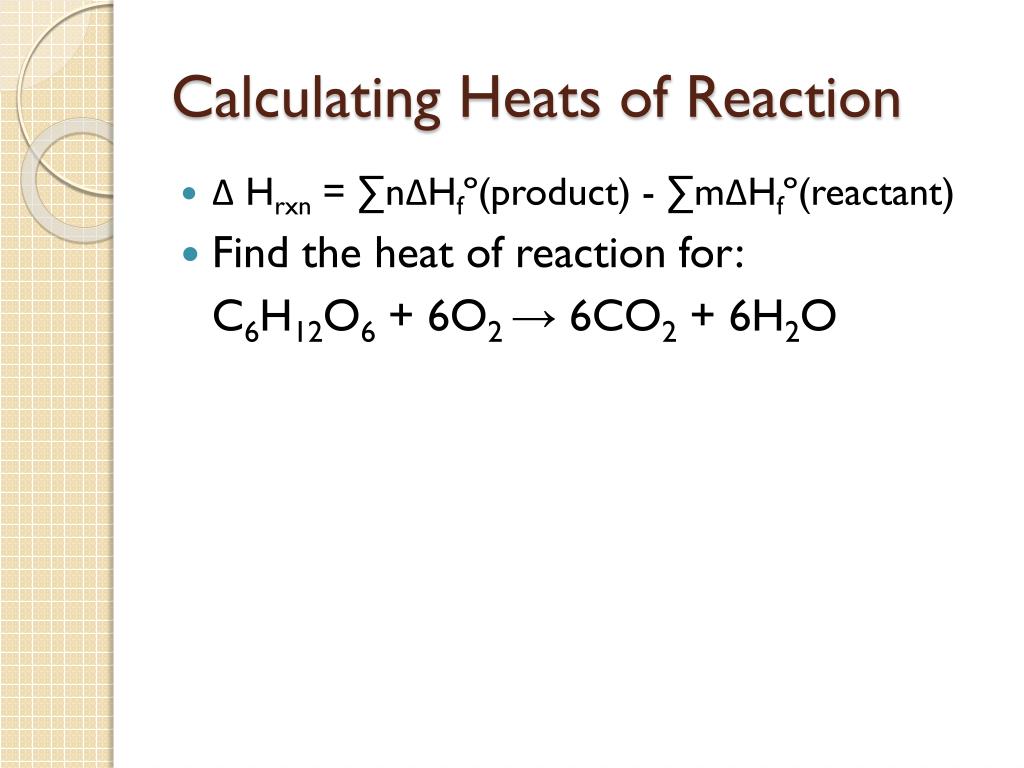
Doceri is free in the iTunes app store.The heat of reaction is also known as Reaction Enthalpy. Conversely, while the reaction absorbs heat ( q rxn > 0), then generate is transmitted from the scales at the system ( question calorimeter < 0) and the temperature of the calorimeter decreases.In the most common approach to coal gasification, coal reacts with steam to produce a mixture of CO and H2 known as synthesis gas, or syngas:Because coal is 70%–90% carbon by mass, it is approximated as C in Equation 7. It is the thermodynamic unit .7 we see that at constant pressure the change in enthalpy, ΔH of the system, is equal to the heat gained or lost.

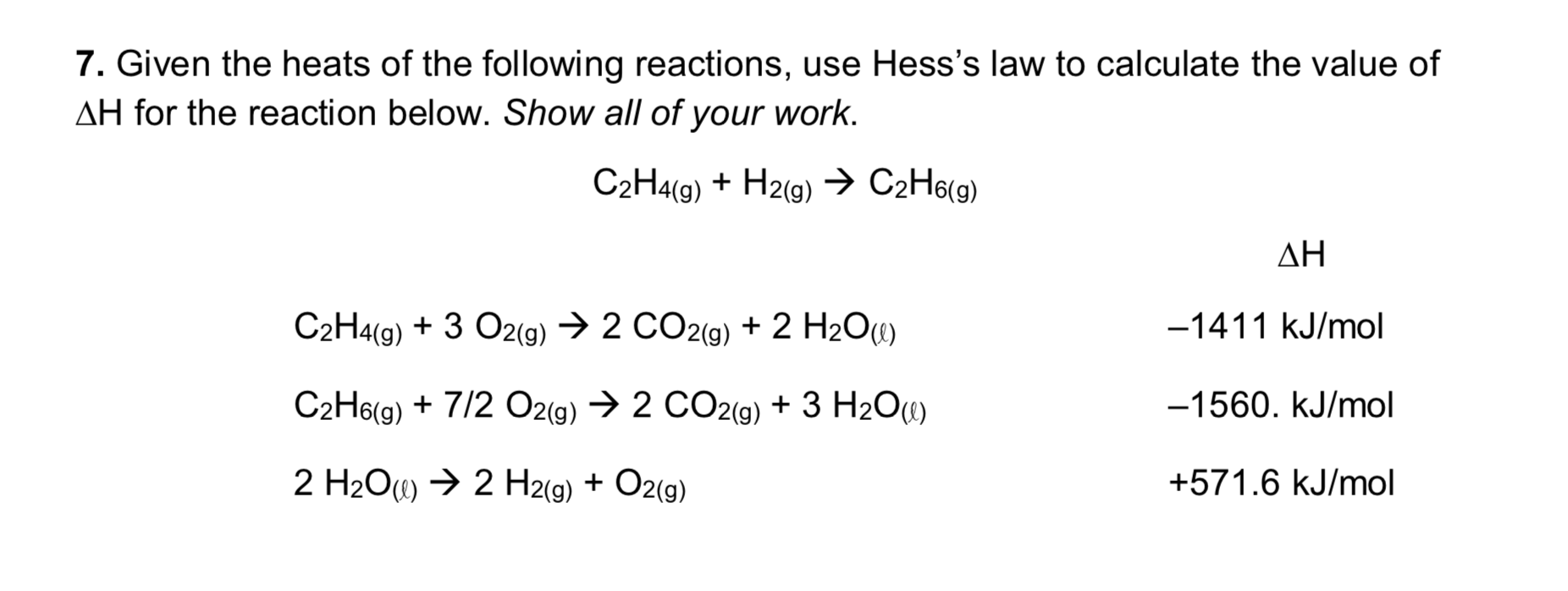
Define bond dissociation energy.This video screencast was created with Doceri on an iPad. Chemical Engineering questions and answers.comSpecific Heat Calculatoromnicalculator. These reactions .Since summing these three modified reactions yields the reaction of interest, summing the three modified ΔH° values will give the desired ΔH°: ΔH ∘ = (+ 102. To avoid confusion caused by differences in reaction conditions and ensure .
- 5700 Xt Stromverbrauch – Kürung der besten Radeon RX 5700 (XT)
- 8 März Ist Frauentag Kinder – Frauentag
- 6 Ssw Keine Anzeichen Mehr : 6 SSW Blutung: Symptome, Risiken, Behandlungen & mehr (2023)
- 7 Years War Definition | Eighty Years‘ War
- 566A Bgb , Mietkaution bei Eigentümerwechsel: Diese Regeln gelten
- 8 Brokate Qigong Youtube _ Qigong der 8 Brokate
- 500 Euro Schein Bäcker _ 500-Euro-Schein wird abgeschafft Volksbank eG
- 52 Kartendeck In Reihe Legen , Patience: Der Kartenspielklassiker für 1 Person
- 7 Inches To Mm | Inch to mm (inches to millimeters)
- 56000 Brutto In Netto : 6000€ Brutto in Netto
- 7 Core Army Values – The Seven Army Values
- 5Pol Stecker : 5 Pol Din Auf Cinch
- 888 Poker Spielplan | Live Poker
- 70 Stvzo Bayern : Bundesportal
- 5 Year Mid Swap Rate , Mid Swap