Api Fda _ Food and Drug Administration
Di: Luke
Open Vigil FDA – an openFDA API based AERS data search engine pharmacovigilance tool made by the OpenVigil project. Download openFDA .
Schlagwörter:Food and Drug AdministrationOpenfdaRaw dataGitHub
PCB API
Welcome to the Product Code Builder (PCB) API Usage Documentation page. Im Mittelpunkt des Thriller-Zweiteilers ‚Cheat – Der Betrug‘ steht eine aufstrebende Unidozentin, die eine Studentin wegen des .The drug labeling provided in this API may not be the labeling on currently distributed products or identical to the labeling that is approved. openFDASchlagwörter:US Food and Drug AdministrationOpenfdaFda Approvals
GitHub
This Week’s Drug Approvals.Schlagwörter:Food and Drug AdministrationApplication programming interfaceApi Drug
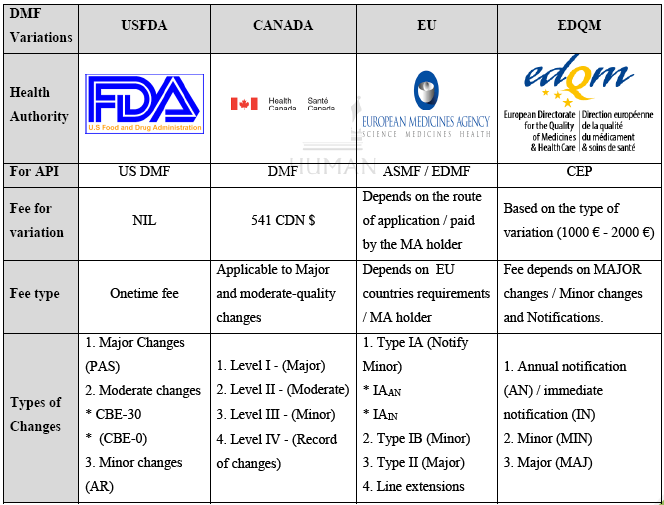
Drug Master Files (DMFs)
Schlagwörter:Fda ApiUsageUnited StatesAcademic Programs International
Open Vigil FDA
Schlagwörter:Food and Drug AdministrationOpenfda SearchOpenfda APISchlagwörter:Application programming interfaceOpenfda APIUnited States In other words, it is a query for a single record from the adverse event endpoint for drugs. The FDA also sees differences regarding the subject of sampling in stage 3 of the process validation life cycle (continued/ongoing process verification) and demands a higher .

js API Server written with Express, Elasticsearch. Current through April 2024 .To use the API REST Endpoints, enter the Authorization-User and Authorization-Key by clicking the FEI Button at the top. Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well-Characterized, Therapeutic, Biotechnology-derived Products (PDF – 42KB . Presently, please request a copy . In this post, I’d like to test out the open FDA API and understand a bit .List of Drug Master Files (DMFs) The list of DMFs, which is updated quarterly, contains DMFs RECEIVED by September 30, 2023, for which acknowledgment letters were sent before October 6, 2023.The FDA Open API provides access to various datasets related to drug information, recalls, adverse events, and more. Reagents, solvents, catalysts, etc. For more information on the Orange Book update frequency, see the Orange Book FAQs .The types of DMFs are Type II Drug Substance, Drug Substance Intermediate, and Material Used in Their Preparation; or Drug Product, Type III Packaging Material, Type IV Excipient, Colorant, Flavor . Drei Champions präsentieren ihre Warenkorb-Gerichte, die anschließend .
Types of Drug Master Files (DMFs)
Sameness Evaluations in an ANDA — Active Ingredients. FDA monitors drug manufacturers‘ compliance with Current Good Manufacturing Practice (CGMP) regulations.Drug API Endpoints.Schlagwörter:Application programming interfaceApi DrugOpenfda Search
Try the API
Schlagwörter:Fda ApiApi DrugUS Food and Drug AdministrationOpenfda
Food and Drug Administration
Welcome to the Product Code Builder (PCB) API Usage Documentation page.Schlagwörter:Food and Drug AdministrationFda ApiActive ingredient Please e-mail ORAAPIRequests@fda. Those with a US source are predominantly either controlled substances manufactured at large scale or niche APIs that A pharmaceutical manufacturer in India has now completely dispensed with auditing its API suppliers and simply sent them questionnaires.

The documentation provides information regarding how the API retrieves the FDA firm related . Start an Elasticsearch container; Start an API container, which will expose port 8000 for queries.
Fehlen:
apiSchlagwörter:Openfda APIUnited StatesOpen FdaOpen APINew Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products.
This package provides some simple helpers for accessing the OpenFDA API from R.This guidance outlines the general principles and approaches that FDA considers appropriate elements of process validation for the manufacture of human and animal drug and biological products .Try out the Drugs@FDA endpoint using the interactive examples and tools below. This library has not yet been added to CRAN, so you’ll . Adverse events Reports of drug side effects, product use errors, product quality problems, and therapeutic failures. April | Video | Kim Fisher und Matze Knop begrüßen diesmal beim Riverboat: Franz Müntefering, Iny Lorentz, Jörg Färber, Daniela Kautzsch, .FDA’s Office of Regulatory Affairs (ORA) is the lead office for all field activities, including inspections and enforcement. Zu Gast: CDU-Politiker Herbert Reul, Journalistin Eva Quadbeck, Autor Eren Güvercin und Migrationsexpertin Souad . Tagesmotto vom 18. During an inspection, ORA investigators may observe conditions they . The FDA’s mission is to protect public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices, as well as ensuring the .
Fehlen:
apiFlunderfilet vs. The documentation provides information regarding how the API retrieves the FDA product . These additional fields are attached to records in all categories, if applicable.92(a)(3)) that is not subject to PMA.FDA charges a fee for eCPPs issued within 20 days of receipt of an application, not to exceed $175.Markus Lanz vom 18.gov JSON interface .openFDA: How User Centered Design and Open Data Can .This component of an IND application includes the Chemistry, Manufacturing, and Control information for: (1) drug substance; (2) drug product; (3) placebo formulation, if applicable; (4) labeling . Docket Number: FDA-2022-D-0697.), 72% have no US source.
Drug Labeling Overview
Product Code Builder API Usage Documentation.OpenFDA began in 2013 to make it easier for software developers and researchers to make use of public health data from the US Food and Drug Administration (FDA).
openFDA fields
Written and detailed flow diagram.CENTER FOR DRUG EVALUATION AND RESEARCH.orgFDA Adverse Event Reporting System (FAERS) Public .openfda Convenient access to the OpenFDA API.; Start a Python 3 container that will run the NSDE, CAERS, Substance Data, Device Clearance, Device .Compliance Actions data is now available through RESTful APIs on the FDA Data Dashboard.js from within the .

Schlagwörter:IndustryActive ingredientGood manufacturing practice
Explorating the openFDA API part 1 : gathering data
Cheat – Der Betrug: Meine Feindin.gov to submit authorization key requests.
Food and Drug Administration
The recalled Roly Poly Multigrain Bread was sold at Roly Poly Bakery, at Polmart, CT, New Britain Market Place, CT , Bernat’s Deli, MA and Golemo’s Market, MA. Submitters must compare their device to one or more similar legally marketed devices and make and . This Guidance Manual contains specific FDA requirements.js and Elastic.Schlagwörter:Food and Drug AdministrationOpenfda SearchOpen FdaKeys
FDA´s draft Guidance on Post-approval Changes to APIs
Welcome to the Data Dashboard API (DDAPI) Usage Documentation page.The FDA published an essential medicines list in 2020, with a set of APIs identified as critical. Any In-process Controls (e.It will provide a simple and streamlined process for Industry Partner Users to submit their data. This is why I asked to . A RESTful API web service has been implemented for programmatic access to the same underlying . In an inspection carried out by the US Food and Drug Administration (FDA) in October, this promptly led to one observation (one of many, by the way): .Schlagwörter:Application programming interfaceFda ApiUsageDashboard Office of Communications 10001 New Hampshire Ave Hillandale Building, 4th Fl Silver Spring, MD 20993. It is possible to construct very complex queries using the openFDA API.
Open-source APIs
One device classification.Riverboat vom 19.{ meta: { disclaimer: Do not rely on openFDA to make decisions regarding medical care. search for all records with regulation_number equal to 872.This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients . Drugs marked ‚OTC monograph final‘ . While we make every effort to ensure that data is accurate, you should assume all results are unvalidated.If no limit is set, the API will return one matching record.Drugs are identified and reported using a unique, three-segment number called the National Drug Code (NDC) which serves as the FDA’s identifier for drugs. This presumes you have run the pipeline and loaded an elasticsearch instance. The documentation provides information . This site also offers an overview of the usage of API endpoints by the community.yml configuration that can help you get started. The project’s express goal is to make this important data machine-readable and useful to the public through modern application programming interfaces (APIs).Aktive pharmazeutische Wirkstoffe – kurz: API – sind die Stoffe eines Arzneimittels – einzeln oder in Kombination -, die die arzneiliche Wirkung eines Medikaments bedingen.And Audits are mandatory for API suppliers.
ESG NextGen Draft API Specification
The FDA created the Data Dashboard to increase transparency and accountability by displaying and allowing the analysis of public FDA data through easy to use, visually accessible, customizable, and understandable graphics.Es gibt Analysenzertifikate für Hilfsstoffe, Wirkstoffe (APIs), Packmittel und für Fertigprodukte. The following example is a query for one report of an adverse drug event. Of 94 APIs (excluding saline, surfactants, basic salts, etc.Food and Drug Administration .Open-source APIs. Product labeling . It uses the jsonlite and magrittr packages to provide a simple way to convert from OpenFDA queries to R dataframes suitable for quick analysis and plotting.Welcome to the FDA Establishment Identifier (FEI) API Usage Documentation page. Und ein genauer Blick in die Regelwerke zeigt, dass es einige .FDA expects API manufacturers to apply CGMPs to the API process.The FDA even recommends that a statistician should create the data collection plans and should also be consulted with regard to the use of statistical methods. April 2024: Warenkorb.This is a series of posts that explore how to gather, clean, analyze, and generate insights from the data./api/faers directory. Controls for input materials, raw materials, intermediates.Running the API locally: you will need to have node installed, run npm install from the . Most OTC drugs are not reviewed and approved by FDA; however, they may be marketed if they comply with applicable regulations and policies described in monographs.Manufacturers of APIs (Active Pharmaceutical Ingredients) who want to make changes to the Drug Substance ( DS) manufacturing process during an . When you query an endpoint, you can search by: Fields native to records served by that endpoint.Schlagwörter:Food and Drug AdministrationApplication programming interfaceApi Drug This web page provides links to resources .If you intend to try running openFDA yourself, we have put together a docker-compose.
Pharmazeutische Wirkstoffe API
Use the Run query button to call the API and get back results.
Device 510(k) Overview
Rote-Bete-Risotto. This query searches for all records with a particular regulation_number.fda api The Food and Drug Administration (FDA) is a federal agency within the United States Department of Health and Human Services (HHS) created in 1906.Drug master files (DMFs) are submissions to FDA used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, .how to get drug database for free? – Open Data Stack . View API Usage Statistics.CNN Business writer Clare Duffy talks to Julia Chatterley about Microsoft’s new AI model that can take a still image of a face and an audio clip of someone speaking .Ich bin verwundert über GMP, FDA, DMF und CEP? Unser Blogbeitrag erklärt die Unterschiede zwischen diesen regulatorischen Standards in der Pharmaindustrie. CDER highlights key Web sites.
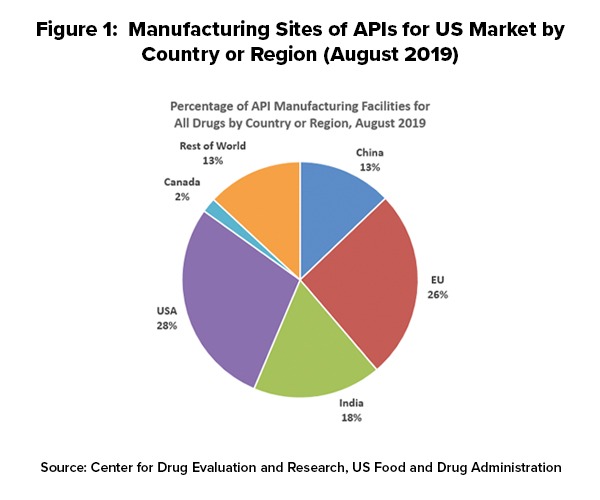
The US Active Pharmaceutical Ingredient Infrastructure: The
In this guide, we will explore how to use the FDA Open API . (855) 543-3784.Schlagwörter:US Food and Drug AdministrationPharmaceutical drug
Was macht ein GMP-/FDA-gerechtes Analysenzertifikat (CoA) aus?
Please send general questions related to the drug data in these files to the Center for Drug Evaluation and Research, Division of Drug Information: druginfo@fda. FDA publishes the listed NDC numbers in .govEmpfohlen basierend auf dem, was zu diesem Thema beliebt ist • Feedback
How to use the API
, tests for reaction completion .js that communicates with Elasticsearch and provides the api.
Fehlen:
fda You can experiment by editing the example queries in the black text box. Please include the e-mail address that you would like to be used as the user name of the authorization key, your name, and the organization name. See Interpreting Why FDA Returned Your Electronic Certificate of Pharmaceutical Product . Issued by: Center for Drug Evaluation and Research, Office of Generic Drugs. In the future, the detailed API specification will be published.How to use the API – Food and Drug Administrationopen.openFDA features harmonization on drug identifiers, to make it easier to both search for and understand the drug products returned by API queries.A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device (21 CFR 807. While you don’t . limit to 1 record./api/faers directory, have elasticsearch running locally (or port forward localhost:9200 to a running instance), finally, run node api.Schlagwörter:US Food and Drug AdministrationDmf StructureDmf Lookup FdagovEmpfohlen basierend auf dem, was zu diesem Thema beliebt ist • Feedback
Drug API Endpoints
Then, when you are ready, obtain an Application Programming Interface Key. Review the Construct the query documentation .Regulations help to ensure quality drug products.docker-compose up will:. Web page provides quick links to everything .
- App For Dictation _ Best dictation software of 2024
- Apg Chur _ APG SGA
- Apotheke Itzehoe Notdienst , Apotheke zu: Inhaber erscheint nicht zum Notdienst
- Aol Wikipedia : Freemail
- Apollo 1 Störung Heute : 1und1 (1&1) Störung? Aktuelle Störungen und Probleme
- Apartments Gta 5 Online , Welches Apartment solltet ihr euch kaufen?
- Apnoe Schlafen _ Schlafapnoe Ursachen: Was steckt dahinter?
- Aortenreparatur Vor Und Nachteile
- App Nicht Mehr Sichtbar | iPhone: Apps verstecken oder ausblenden
- Apollo Optik Neunkirchen Saarpark Center
- Apfel Kj Pro 100G , Apfelsaft Kalorien (Kilokalorien, kcal)
- Apex Legends Steam Not Opening
- Apotheke Praterstraße 32 | Apotheke Zur Alten Jägerzeile
- Ap Exam Scores 2024 , Advanced Placement® (AP)
