Butane Conformation Chemistry – Organic Chemistry 1: An open textbook
Di: Luke
This indicates a general principle, that Big-Big .This wedge and dash drawing represents one conformation of ethane. Conformations are different arrangements of atoms that result from bond rotation. It is called the anti conformation.Conformations of butane., 1, 3 or 5), and the least stable is eclipsed (i.
Conformations of ethane, propane & butane
Hence, the relative stabilities of the various conformations of ethane are: Staggered > skew > eclipsed.The full rotation will be .Autor: The Organic Chemistry Tutor It explains how to draw the newman projections of ethane, butan.
Organic Chemistry 1: An open textbook
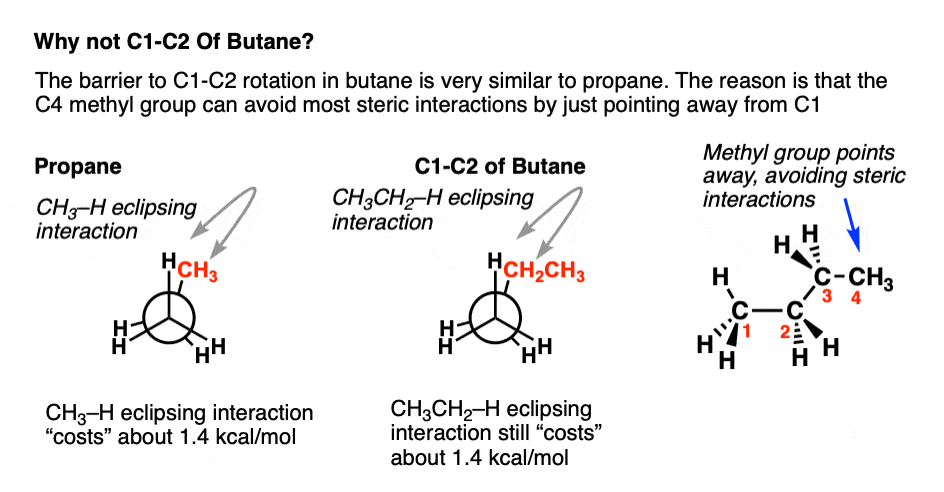
Learning Objective.In butane, for instance, the lowest-energy arrangement, called the anti conformation, is the one in which the two methyl groups are as far apart as possible—180° away from .This page titled 12. Before we begin our exploration of stereochemistry . However, the energy barrier in propane is 14 kJ mol . The boat conformation comes from partial C-C bond rotations (only flipping one carbon up to convert the chair to a boat) of the chair conformation, and all the carbons still have 109. There are three C-C bonds in butane, and rotation can occur about each of them. A plot of potential energy against rotation about the C(2)–C(3) bond in butane is shown below. The fully eclipsed conformation is clearly the highest in energy and least favorable since the largest groups are interacting directly with each other.) 4: Organic Compounds – Cycloalkanes and their Stereochemistry 4. There are now three rotating carbon-carbon bonds to consider, but we will focus on the middle bond between C 2 and C 3. As it continues to rotate, it . It explains how to draw the eclipsed and staggered conformations of .uk and the practie pr.Konformationen entstehen durch Rotation um Einfachbindungen. There is electron repulsion, known as torsional strain, between the overlapping electrons clouds of the two σC−H σ C − H bonds.It explains how to draw the newman projections of ethane, butane, and 2,3-dimethylpentane. For example, ethane (C2H6), there would be no reason to differentiate.Butane conformers – Visualize Organic Chemistry.The staggered form of butane — with a dihedral angle of 180° — has the lowest energy and is the most stable form.
Butane conformers
Geschätzte Lesezeit: 7 min
Newman Projection of Butane (and Gauche Conformation)
3: Conformations of Cycloalkanes is shared under a CC BY-NC-SA 4. In this conformation, the carbon-carbon ring bonds are able to assume bonding angles of ~111 . Here we have the staggered conformation of ethane, looking at it from a wedge and dash perspective.Below are two representations of butane in a conformation which puts the two CH3 groups (C1 and C4) in the eclipsed position, with the two C-C bonds at a 0o dihedral angle. We know that there’s free .In ethane, for instance, rotation around the C–C bond occurs freely, constantly changing the spatial relationships between the hydrogens on one carbon and those on the other ( Figure 3.The staggered conformation is lower in energy than the eclipsed conformation.5º bond angles, so there are no angle strains.

As rotation around the C2–C3 bond occurs, an eclipsed conformation is reached where there are two CH 3 H interactions and one H H interaction.Conformations of Butane.Purdue: Chem 26505: Organic Chemistry I (Lipton) Chapter 3. The hydrocarbon butane has a larger and more complex set of conformations associated with its constitution than does ethane. Like charges repel, so when they are close together, there is an increase in energy.What does degenerate mean in this sense? 4:05I believe it means that the two eclipse conformations have the same energy. If we rotate this a little bit, we’ll see the staggered conformation of ethane .Butane is an alkane with the presence of C-C bonds.
Conformations of Butane

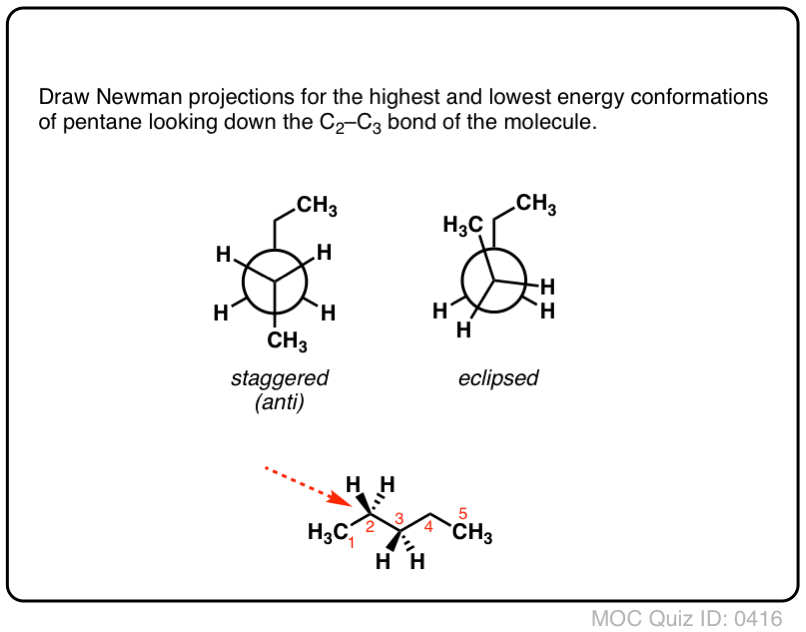
A Newman projection, useful in alkane stereochemistry, visualizes the conformation of a chemical bond from front to back, with a line representing the front atom and a circle representing the back carbon.Ein ekliptisches Konformer in Sägebock-und Newman-Projektion Eine gestaffelte Konformation in Sägebock- und Newman-Projektion. [ Note 6 ] This barrier isn’t big enough to prevent “free” rotation about the C-C bond, but it does mean that the staggered and eclipsed conformations will be present in different .Write their chemical symbols on the left, right, top, and bottom of the dot and the circle.So let’s watch the video and look at the different conformations of ethane. Pentane and higher alkanes have conformational preferences similar to ethane and butane. you start counting carbons from the.Now let’s consider butane, with its four-carbon chain.
Conformational analysis of alkanes
I am not in school right now, but i am studying at home.Figure 4: Conformations of butane: fully eclipsed, gauche, eclipsed, and anti. In the staggered conformation, electrons can delocalize from the σC−H σ C − H into the σ∗C−H σ C − H ∗.Four Conformers of Butane: Potential Energy Profile for Butane Conformers; Summary of Conformational Stereoisomerism.Organic Chemistry (Morsch et al. Conformation of Propane: The next higher member in alkane series, propane has also two extreme conformations: staggered and eclipsed conformation.Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position. View Author Information.
Conformations of chain alkanes. The lowest energy conformation of ethane, shown in the figure above, is called the ‘staggered’ conformation, in which all of the C-H bonds on the front carbon are positioned at dihedral angles of 60°relative to the C-H bonds on the .Conformational analysis is a comparatively new area of organic chemistry that has been developed well after the theories of organic reactions, bonding in organic compounds and stereochemistry. It refers to the arrangement of atoms where the carbon-carbon (C-C) bonds are aligned directly with each other, resulting in increased torsional strain. This chair conformation is the lowest energy conformation for cyclohexane with an overall ring strain of 0 kJ/mol. The carbon atom at the front is called proximal, while the atom at the back is called distal. Roberts and Marjorie C.11: Conformations of Butane. The lowest-energy arrangement, called the antiperiplanar (or anti) conformation, is the one in which the two large methyl groups are as far apart as . The Newmen projections are shown bellow. The different arrangements of atoms .In the case of ethane, conformational changes are very subtle, but in others they are more obvious. I was on chemguide. However, the hydrogens on the base of the boat are all in eclipsed . This means that there is a small barrier to rotation of about 3.Geschätzte Lesezeit: 7 min And if we stare down .Butane shows us that the eclipsing of two methyl groups + two H groups destabilizes more than eclipsing of two H||CH 3 pairs.
5: Conformations of Higher Alkanes is shared under a license and was authored, remixed, and/or curated by LibreTexts.Figure 3: Conformations of butane: fully eclipsed, gauche, eclipsed, and anti.Conformations of chain alkanes | Organic Chemistry 1: An open textbook. This conformation is stabilized due to the absence of steric repulsion between the largely spaced out methyl groups.What videos is he referring to at 6:09?He was talking about conformational analysis of ethane and propane Those are the last two videos prior to this one-conformational analysis of butan.Staggered and Eclipsed Conformations of Butane
Conformational analysis of butane (video)
At 180 degrees, the molecule is staggered again and has settled into a regular, zig-zag, letter Z shape. With cases like this, space-filling models .The eclipsed conformation of butane is a significant concept in organic chemistry. Rotation of the groups around a single bond can lead to conformations, which represent different three-dimensional shapes available to a single molecule.At room temperature, butane is most likely to be in the lowest-energy anti conformation at any given moment in time, although the energy barrier between the anti and eclipsed conformations is not high enough to prevent constant rotation except at very low temperatures. However, if we consider the rotation about the C2-C3 bond, the situation will be . Now let us consider butane, a slightly larger molecule. This is the highest energy conformation .
A Theoretical View on the Conformer Stabilization of Butane. Normally, when we rotate the molecule of butane at the axis of the C-C bond, it shows different conformation . Stereochemistry 3.What is the difference between torsional strain and steric hindrance?Torsional strain is the increase in potential energy in the eclipsed Newman projection due to the repulsion between the electron clouds of 2 neighb.Four Conformers of Butane: The following diagram illustrates the change in potential energy that occurs with rotation about the C2–C3 bond. Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position, with the two C-C bonds at a 0 o dihedral . The staggered conformation of butane is the lower-energy and more stable conformation due to minimized steric hindrance between the two methyl groups.
As the molecule rotates, it adopts the relatively stable gauche conformation. Die Konformation beschreibt in der Chemie die räumliche Anordnung der Atome eines Moleküls bei gegebener Konstitution und Konfiguration.0 license and was authored, remixed, and/or curated by John D. Each dihedral angle tries to adopt a staggered conformation and each internal C-C bond attempts to take on an . The model on the right is shown in conformation D, and by clicking on any of the colored data points on the potential energy curve, it will change to the conformer corresponding to that point. There is no torsional strain, . The two conformations of ethane, . A plot of potential energy against rotation about the C (2)–C (3) bond in butane is . Contributors; The hydrocarbon butane has a larger .3a Boat conformation of cyclohexane.A conformation analysis is an investigation of the energy differences and relative stabilities of the different conformations of a compound. For this reason (and also simply for ease of drawing), it is conventional to .The staggered form of butane in which the bulky methyl groups on the two carbons are placed on opposite sides, that is, at a dihedral angle of 180°, is the lowest energy, most stable form — called the anti conformer.
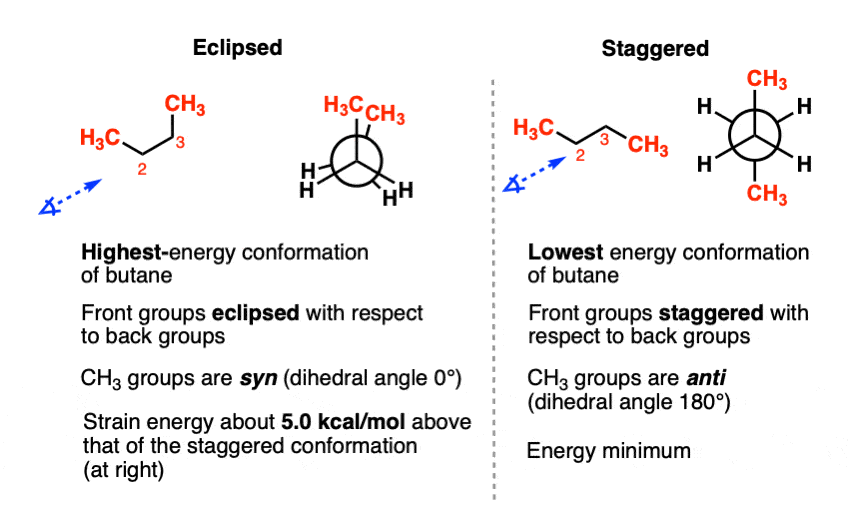
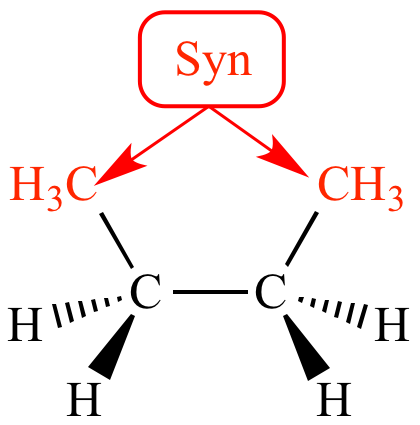
The increased stability of anti .2 Conformations of Alkanes .Video transcript.In this article: introducing conformational isomers, staggered and eclipsed conformations, the Newman projection, the dihedral angle (torsion angle), and the . In IUPAC naming, alcohol takes priority in naming over double bonds (i.In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on . Conformations are the different 3-dimensional .Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position, with the two C-C bonds at a 0 o dihedral angle.Conformational analysis is the study of the different energy levels associated with the different conformations of a molecule.4 Conformation Analysis of Butane. The conformational possibilities increase as alkanes become larger.Are there similar terms for anti and gauche when talking about the eclipsed confirmation?Yes, but only when you have a unique functional group to differentiate them. Comparing 1, 3 and 5, we see that 1 has two “bad” gauche interactions, whereas 3 and 5 have only one gauche interaction; thus 3 and 5 are both equally stable, and they are the most stable conformations for 2 .It depends where the double bond is. – [Voiceover] Here we have the butane molecule, and this is carbon one, carbon two, carbon three, and finally carbon four. The conformations of butane above show the staggered . If we pick up C1-C2 (or C3-C4) for the study, the situation is almost the same as propane, with the ethyl CH 2 CH 3 group replace the CH 3 group. Click the structures and reaction arrows to view the 3D models and animations respectively.For this class, we will always find that the most stable conformation is staggered (i. interpret and draw the rotation about a carbon-carbon single bond using Newman projections and sawhorse structures. It was only in the second half of twentieth century that its importance was fully recognized and its central role with respect to bonding, reactivity . Butane exhibits two types of staggered conformations: the anti (4) and the gauche (2) conformations.
Anti, Gauche, and Eclipsed Conformations of Butane
6 Rotation occurs around the carbon–carbon single bond in ethane because of σ bond cylindrical symmetry.5: Conformations of Cyclohexane . Newman projection of butane in the more-stable eclipsed conformation. Butane (CH 3 CH 2 CH 2 CH 3) has four .In butane, for instance, the lowest-energy arrangement, called the anti conformation, is the one in which the two methyl groups are as far apart as possible—180° away from each other. The equilibria (relative stabilities) and equilibration (rate of interconversion) of the rotational conformations of ethane and butane were discussed previously. Butane, a four-carbon alkane, exhibits flexibility due to the rotation around its C-C bonds.Video ansehen23:36This organic chemistry video tutorial provides a basic introduction into newman projections.
- Butterfisch Beschwerden – Blasenentzündung: Die richtige Ernährung beugt vor und hilft
- Buxbaum Ersatz _ Buchsbaumersatz: Robuste, immergrüne Alternativen zum Buchsbaum
- Busaras Drogheda | Busáras nach Drogheda per Zug, Bus, Taxi oder Auto
- Bvb Damenfüßball Bezirksliga 4
- Bwb Zählerstand Melden | Anmeldung bei eToro
- Bw Secure Kreditkartenzahlung | BW-Secure App funktioniert nicht mehr!
- Business Englisch Zertifikat 2024
- Bwl Studieren Ohne Abitur – Kein Abitur und trotzdem studieren?
- Buslinie 21 Hildesheim _ Buslinie 25 , Hildesheim
- C Apotheke : C+ Kapseln: Risiken und Alternativen