Dot Structure For Fluorine _ How to Draw the Lewis Dot Structure for F- (Fluoride ion)
Di: Luke
Note that hydrogen is often shown in both group 1A .
How to Draw the Lewis Dot Structure for F2 : Diatomic Fluorine
Answer : Electron-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. Thus, In the production of flat panel displays, MEMs, and .A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Fluorine gas). We can show the electron configuration for fluorine using a Lewis diagram (or electron-dot structure), named after the American chemist G. The electron dot diagram also called lewis’s structure which represents the valence electrons of atoms. lone pair: two (a pair of) valence electrons that are not used to form a covalent bond. März 2017What is electron configuration of fluoride ion? | Socratic4. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 4. The Lewis electron dot structures of a few molecules are illustrated in this subsection. The arrangement of the valence electrons for oxygen and hydrogen when they bond show that the Lewis dot structure represents. In a Lewis diagram, the electrons in the valence shell are . Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).comEmpfohlen auf der Grundlage der beliebten • Feedback For fluorine atom: ⇒ Valence electrons of fluorine = 7.0 license and was authored, remixed, and/or curated by LibreTexts.comLewis Dot Diagrams of the Elements – STEM Sheetsstemsheets.Two fluorine atoms gain one electron each to become two -1 ions (anions). Note that the lone pairs (non-bonding) pairs of electrons are still shown around the fluorine atom in the valence structure.Lewis Structure (electron dot diagram) for hydrogen fluoride OR . I am sure you will definitely learn how to draw lewis structure of IF2- ion).
Lewis Structure of CBr2F2 (With 6 Simple Steps to Draw!)
Rubidium transfers its one outer shell .How to draw Lewis Dot Structure Detailed explanation, .Let’s explore the Lewis structure of boron trifluoride, BF3.Fluorine atom lewis dot structure. The BF3 molecule comprises a central boron (B) atom and three surrounding fluorine (F) atoms.In the case of fluorine, the Lewis structure shows a single dot for each valence electron, placed on one of the four sides around the symbol F.
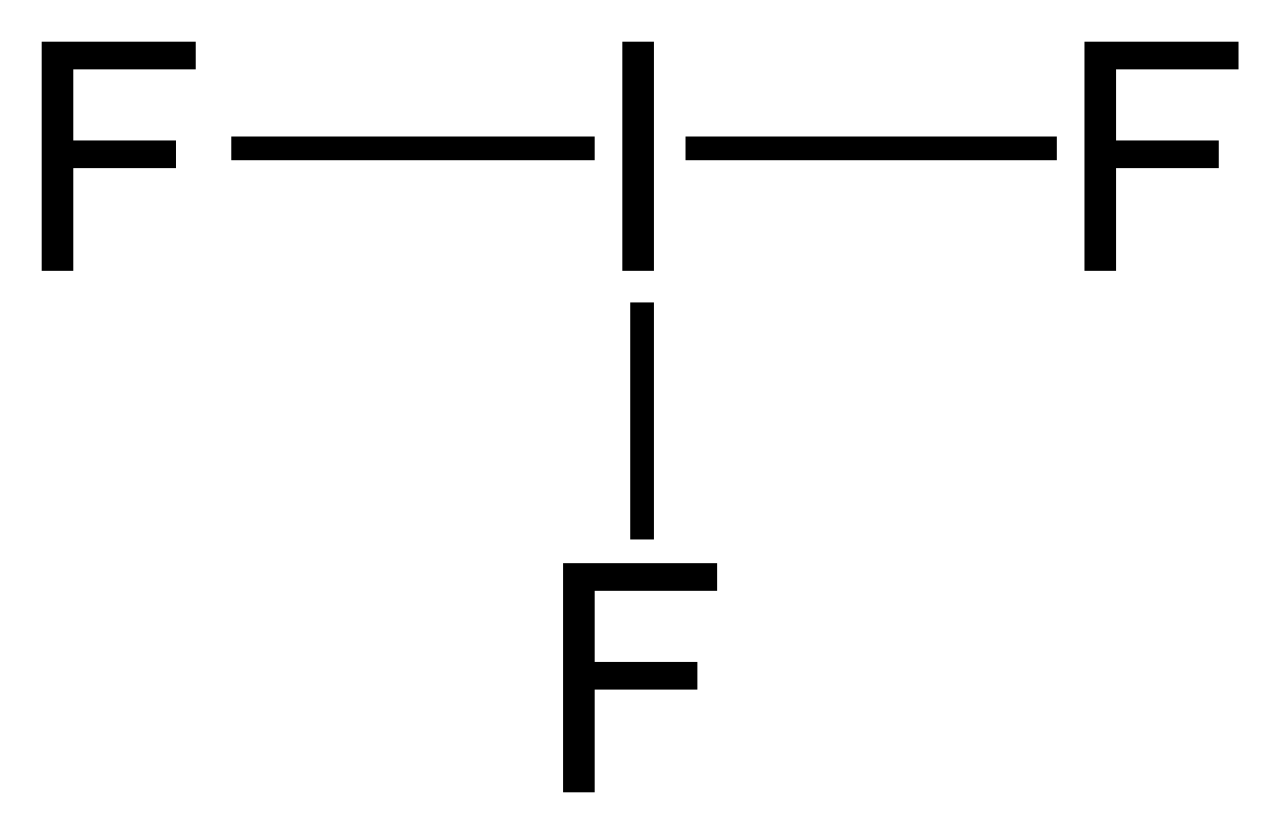
Lewis Structure of IF2- (With 5 Simple Steps to Draw!)
Draw electron dot structure of CO2,H 2O,F 2,N 2. The reactivity of the . So, just represent these 7 valence electrons around the Fluorine atom as a dot. for the one fluorine atom.Draw the electronic dot structures for :(a) ethanoic acid(b) H2S (c .For the F2 structure use the periodic table to find the total number of v.The Nitrogen atom has 2 lone pairs, and both the Fluorine atom has 3 lone pairs. Lewis symbol: symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion.In the case of fluorine, there are 7 valence electrons in the outermost energy level.Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. Atomic Symbol: F; Atomic Number: .The Iodine atom as well as both the Fluorine atoms have 3 lone pairs. (Note: Take a pen and paper with you and try to draw this lewis structure along with me.comLewis Dot Structures – Chemistry LibreTextschem. We’ll use a Bohr diagram to visually represent where the electrons.Electron dot diagram of Fluorine atom.Draw the electronic dot structure of ethane molecule (C2H6)Weitere Ergebnisse anzeigen Each fluorine atom has three lone pairs.In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. In the case of fluorine, the Lewis structure shows a single dot for each valence electron, placed on one of the four sides around the symbol F.

Lewis Dot Structures should be review from your preparatory chemistry course.
Drawing dot structures (video)
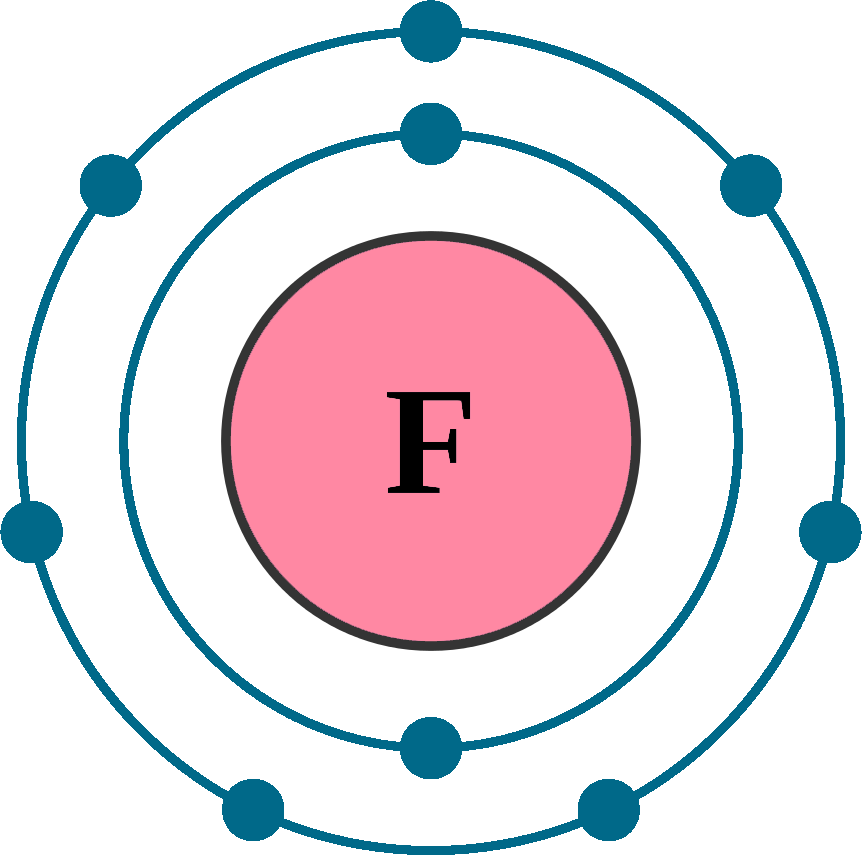
In this video we’ll look at the atomic structure and Bohr model for the Fluorine atom (F). Fluorine, a group 7A element, is the provided element.
The Dot Diagram of Fluorine: Understanding its Atomic Structure

Figure 2: Electronic configuration of fluorine.In the lewis structure of Nitrogen trifluoride (NF 3), there are three N-F bonds and one lone pair on nitrogen atom. Home | Wissenschaft | Chemie. Fluoride (F): – Place one dot around the symbol of fluorine, representing its valence electron. Learning Objectives.Fluorine Lewis dot structure: Drawing, Several Compounds And Detailed Explanations – TechieScience. I am sure you will definitely learn how to draw lewis structure of . Draw resonance structures of some molecules.Fluorine, for example, with the electron configuration [He]2s 2 2p 5, has seven valence electrons, so its Lewis dot symbol is constructed as follows: Figure 8. Since it is bonded to only one carbon atom, it must form a double bond. Lewis Structure of CO2. Valence electrons occupy the highest energy level (also . In OF2, we have: Oxygen (O) – 6 valence electrons.}F}}\mathbf{:}\nonumber \] . Fluorine (‘group 17’ element) has total nine electrons with electronic configuration:1s2 2s2 2p5. Explanation: And thus the neutral atom has 7 valence electrons. The Nitrogen atom has -1 formal charge. Step #1: Calculate the total number of .In the structures, the electrons are shown as dots.
OF2 (Oxygen Difluoride) Lewis Structure
F2 Lewis Structure: How to Draw the Lewis Dot Structure for F2
Draw the Lewis dot structure of a given molecule or ion. In this section, we will outline several key concepts applied in working with Lewis Dot Structures.Infact, I’ve also given the step-by-step images for drawing the lewis dot structure of CBr2F2 molecule. 2016How would you represent potassium and bromine using an . Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Fluorine is in Group 17 of the Periodic Table . Fluorine atom requires one electron to make energetically stable outer orbit structure. Fluorine (F) – 7 valence electrons each (2 atoms) Total valence electrons = 6 + 7*2 = 20. Fluorine is a ‘group 17’ element. Each step of drawing the lewis structure of NF 3 is explained in .These are held t. a linear shape.What is the Ionic Bonding between Potassium and Fluorine . Click here:point_up_2:to get an answer .
How to Draw the Lewis Dot Structure for F- (Fluoride ion)
How to Draw the Lewis Dot Structure for F2 : Diatomic Fluorine. Now it is possible for some of the .
Lewis Structures (electron dot diagrams) Chemistry Tutorial
Lewis-dot structure is a diagram that depicts the valence electrons that are present in an element.Fluorine and neon have seven and eight dots, respectively: With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its .It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds.One magnesium atom loses two electrons, to become a +2 ion (cation).Fluorine is a Group 7A element and has seven valence electrons. Of course the elemental . Lewis, who proposed the concepts of electron shells and valence electrons.Fluorine (F2 2) Molecule Lewis Structure.Lewis Structure Examples. A larger outer circle has one red dot on, representing the second shell with one electron. Begin by counting the total valence electrons in the molecule. As we know that, carbon has ‚4‘ valence electrons, hydrogen has ‚1‘ valence electron, sulfur and oxygen has ‚6‘ valence electrons and fluorine has ‚7‘ valence electrons. Rubidium has one outer orbital electron which is in 5s; as a ‘group 1’ element.

In outermost shell of Fluorine, it . Question 5 (d) Draw the electron dot structure for F2. Fluorine is the most electronegative element because it has 5 . Counting Valence Electrons.Rubidium Fluoride lewis dot structure. Of course the elemental form is bimolecular.Fluorine’s atomic electron configuration is 1s 2 2s 2 2p 5 (see Figure 2).We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. This sharing of electrons allowing atoms to stick together . And thus the neutral atom has 7 valence electrons. 1: shows the Lewis symbols for the elements of the third period of the periodic table.It is found in the -1 oxidation state, except when bonded to another fluorine in F 2 which gives it an oxidation number of 0. Nitrogen atom (Group 15) has 5 valence . These electrons are represented by placing dots around the symbol of the element.To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons.⚛ Lewis electron dot structures A dot is used to represent a valence electron.
BF3 (Boron trifluoride) Lewis Structure
Lewis used the . ⇒ Lone pair electrons on fluorine = 6 Which do you think would be bigger; fluorine atom or fluoride ion?
F2 Lewis Structure (Fluorine Gas)
Atomic Structure (Bohr Model) for Fluorine (F
a trigonal planar shape. In a water molecule, oxygen has two shared pairs of electrons and two unshared pairs of electrons, which bond with one valence electron in hydrogen. Fluoride ion (F1-): – Since there is an extra electron, place brackets around the symbol and include a negative charge (-) outside the brackets.Fluorine and neon have seven and eight dots, respectively: \[\mathbf{:}\mathbf{\ddot{\underset{. 2015Weitere Ergebnisse anzeigenFluorine is in Group 17 of the Periodic Table.The Lewis structure of CHF 3 consists of a carbon (C) atom at the centre, which is bonded to one hydrogen (H) atom and three atoms of fluorine (F).A step-by-step explanation of how to draw the RbF Lewis Dot Structure.Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. It has seven electrons in last shell which are in 2s and 2p orbital.To create the Lewis structure of OF2, follow these steps: 1. 6 Steps to Draw the Lewis Structure of F2. Step-by-Step Guide to Drawing the .The fluorine atom is at the center and it is surrounded by 1 oxygen atom and one O-H group.orgEmpfohlen auf der Grundlage der beliebten • Feedback Let’s draw and understand this lewis dot structure step by step. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. The central atom of this molecule is carbon.A step-by-step explanation of how to draw the F- Lewis Dot Structure. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.2: Interpreting Lewis Structures is shared under a CC BY-NC-SA 4.Electron Configuration Chart of All Elements (Full Chart)periodictableguide. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Lewis structure of CBr2F2 contains a single bond between the Carbon-Bromine atoms and Carbon-Fluorine atoms.Now we can draw the Lewis dot structures: 1.Hi Everyone! In this video, we will help you find out the Lewis Structure of Fluorine gas molecules. Using Lewis Dot Symbols to Describe Covalent Bonding.For the F- Lewis structure use the periodic table to find the total number of valence el. Principal Energy Levels. Lewis structure of fluorine molecule contains only one F-F bond .Let’s count the formal charge on the fluorine atom first, both fluorine atoms in the BeF2 Lewis structure(4th step) have the same bonded pair and lone pair, so, just count the F. All 4 electron density regions are comprised of bond pairs; thus, there is no lone pair of . Atomic Symbol: F; Atomic Number: 9; Valence Electrons: 7 Fluorine combines with the noble gases argon, .Note that Diatomic Fluorine is often called Molecular Fluorine or ju. The Iodine atom has -1 formal charge.A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Diatomic Fluorine). Fluorine is a diatomic molecule and contains only two fluorine atoms. Boron resides in group 13 of the periodic table, boasting three valence electrons, while each fluorine atom originates from group 17, contributing seven valence electrons.Note that an electron dot structure for fluorine should not be placed on either the top or right sides of sulfur’s electron dot structure, as stable electron pairs already exist in each of .The dot diagram, also known as the Lewis structure, illustrates the arrangement of these valence electrons around the atomic symbol. I am sure you will definitely learn how to draw lewis structure of HOFO).For RbF we have an ionic compound and we need to take that into account when we draw th. It has a very simple structure and with our step-by-step. Assign formal . Juli 2018How would you show the bonding of Hydrogen and Oxygen .Fluorine is an element with a 17-atomic-number and ‚7‘ valence electrons.Lewis Dot Structures.So for elements like carbon, nitrogen, oxygen, fluorine, understanding the octet rule is going to help you when you’re drawing dot structures.
The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which . – The extra electron pairs with one of the . Oxygen contains 6 valence electrons which form 2 lone pairs. This suggests the best Lewis structure has three B–F single bonds and an electron deficient boron.2: Electron-Dot Model of Bonding – Lewis Structures. There are a total of 4 electron density regions around the central C-atom in the CHF 3 Lewis structure. Example: Lewis Structure for ammonia, NH 3.
- Dopgas Tankstelle Österreich , Liste der Autogas Tankstellen in Österreich
- Doppelwandiger Öltank Vorschriften
- Doxycyclin Tabletten Anwendung
- Dortmund Hauptbahnhof Parken _ Anreise & Parken
- Douglas Camouflage Make Up | Camouflage Make-up
- Dove Cameron Verlobung : Sind Dove Cameron und Mitchell Hope zusammen?
- Dorint Hotel Jena – Enjoy comfort and elegance at the Dorint Hotel Esplanade Jena
- Doxepin Abhängigkeit | Doxepin-Abhängigkeit und Nebenwirkungen in Deutschland
- Dora Games Free _ Dora the Explorer: La Casa de Dora
- Dove Styling Products _ Dove Hair Products
- Dove Men Care Invisible Dry , DOVE Men+Care Invisible dry dezodorant 150ml
- Dota 2 Einsteiger Guide : Steam Community::Dota 2
- Dosentelefon – Telefonbuchse anschließen
- Dot Com Bubble Crisis : Dotcom Blase: Absturz der Tech-Aktien erinnert an Börsencrash