Element Electronegativity Chart
Di: Luke
For chemistry students and teachers: The tabular chart on the right is arranged by electronegativity.0) and cesium and francium are the least electronegative elements (EN = 0. 2: The electronegativity scale was developed by Nobel Prize winning American chemist .Truro School in Cornwall.12: Electronegativity. Thus, we can say the electronegativity of an atom is of relative value . The higher the electronegative of an element, the more .Schlagwörter:Electronegativity ChartElectronegativity Table of ElementsSchlagwörter:Electronegativity Table of ElementsElectronegativity Periodic Table
Electronegativity Definition and Trend
Check out the electronegativity values of elements here.Schlagwörter:Electronegativity ChartElectronegativity of Electrons Francium has an electronegativity . This ScienceStruck article brings you the electronegativity chart to get a better understanding of the relationship between two elements. The electronegativity chart describes how atoms can attract a pair of electrons to itself and by looking at the chart you can easily determine the . On the Pauling scale, fluorine is the most electronegative element (EN = 4.
Electronegativity Chart of Elements: Periodic Trend
click on any element’s name for further chemical properties, environmental data or health effects. This list can also be used to get whether the resulting molecule will . Because Cl lies above and to the right of Se, we can predict that χ Cl > χ Se.Electronegativity Chart of All Elements (All Values Inside) March 23, 2023. The Pauling scale is the most commonly used. The idea is to distill all the knowledge of chemistry into a single number, a scale, that ranges from 0 to 4. Understanding electronegativity helps us comprehend why certain elements are more likely to form specific types of bonds.An element’s electronegativity provides us with a single value that we can use to characterize the chemistry of an element.2: 2: Electronegativity of Helium (He) N/A: 3: Electronegativity of Lithium (Li) 0.Schlagwörter:Electronegativity Table of ElementsElectronegativity Periodic Table
Electronegativity
This value is an essential quantity as it determines the type of chemical bond formed between two atoms. When two atoms combine, the difference between their electronegativities is an .Linus Pauling described electronegativity as “the power of an atom in a molecule to attract electrons to itself.But if you want to see the electronegativities of all the 118 elements, then visit: Electronegativity chart of all the elements. Rather, it depends on the other atoms in a molecule and also depends on the nuclear charge and number of electrons. Electronegativity is a concept developed by Linus Pauling, a rather famous chemist who won the Nobel Prize twice (once for chemistry, once for peace). The electron affinity of an element is a measurable physical quantity, namely, the energy released or absorbed when an isolated gas-phase atom acquires an electron, measured in kJ/mol.Table of Electronegativities (Pauling Scale) Alan D. Electronegativity of an atom is a relative value of that atom’s ability to attract election density toward itself when it bonds to another atom. An interactive version of this table may be found here .2: Electronegativity is shared under a CC BY-NC-SA 4.Find a periodic table of all elements with respective eletronegativities. Electronegativities are used to determine the polarity of covalent bonds. On the periodic table, electronegativity generally increases as you move from left to right .Schlagwörter:Electronegativity of ElectronsLibreTexts Ionization, Radius, Hardness, Modulus, Density, Conductivity, Heat, Abundance, Discovered. Print out a PDF version of electronegativity chart to study and for reference.16 Electronegativity (Pauling Scale) 1 H Hydrogen 2. Because Sr lies far to the left of the other elements given, we can predict that it will have the lowest electronegativity. (Make sure you visit this, because I have mentioned the electronegativities along with the images for each element).Schlagwörter:Chemical ElementsElectronegativity Chart with ValuesChart of Periodic Table Trends. Please note that the elements do not show their natural relation towards each other as in the Periodic system. Fluorine (the most electronegative element) is assigned a value of 4.Schlagwörter:Electronegativity ChartElectronegativity ValuesChemical Elements We also acknowledge previous National Science Foundation support under .Electronegativity Trends.The electronegativity chart or table shows the elements present on the right top side (r-t) of the periodic table (p.
Electronegativity Definition and Trend
Elements are grouped according to similar electronic structure, which makes these recurring element properties readily apparent in the .” 1 Basically, the electronegativity of an atom is a relative value of that atom’s ability to attract election density toward itself when it bonds to another atom. Electronegativity, on the other hand, .Overview
Periodic Table
The first chemical element is Actinium and the last element is Fluorine.0, and values range down to cesium and francium which are the least electronegative at 0.Wel
PERIODIC TABLE OF ELEMENTS
Schlagwörter:LibreTextsThe Most Electronegative Element Unit 2: Periodic Properties of the Elements.Schlagwörter:Electronegativity ChartElectronegativity of ElectronsPeriodic Trends
Electronegativity Chart PDF
Pauling defined electronegativity as “the power of an atom in a molecule to attract electrons to itself. The unity used for the electronegativity is Pauling. Looking at the chart above, you can see certain electronegativity trends on the electronegativity chart.PERIODIC TABLE OF ELEMENTS Electronegativity 17 Atomic Number Cl Symbol Chlorine Name 3.Electronegativity is a dimensionless number; the greater the electronegativity value, the greater the attraction for shared electrons. The difference in the electronegativity values of two elements is known as .While there are charts of electronegativity values for elements of the periodic table, there is no true single electronegativity value for an atom. It is the measure of an atom’s ability to attract electrons in a covalent bond.
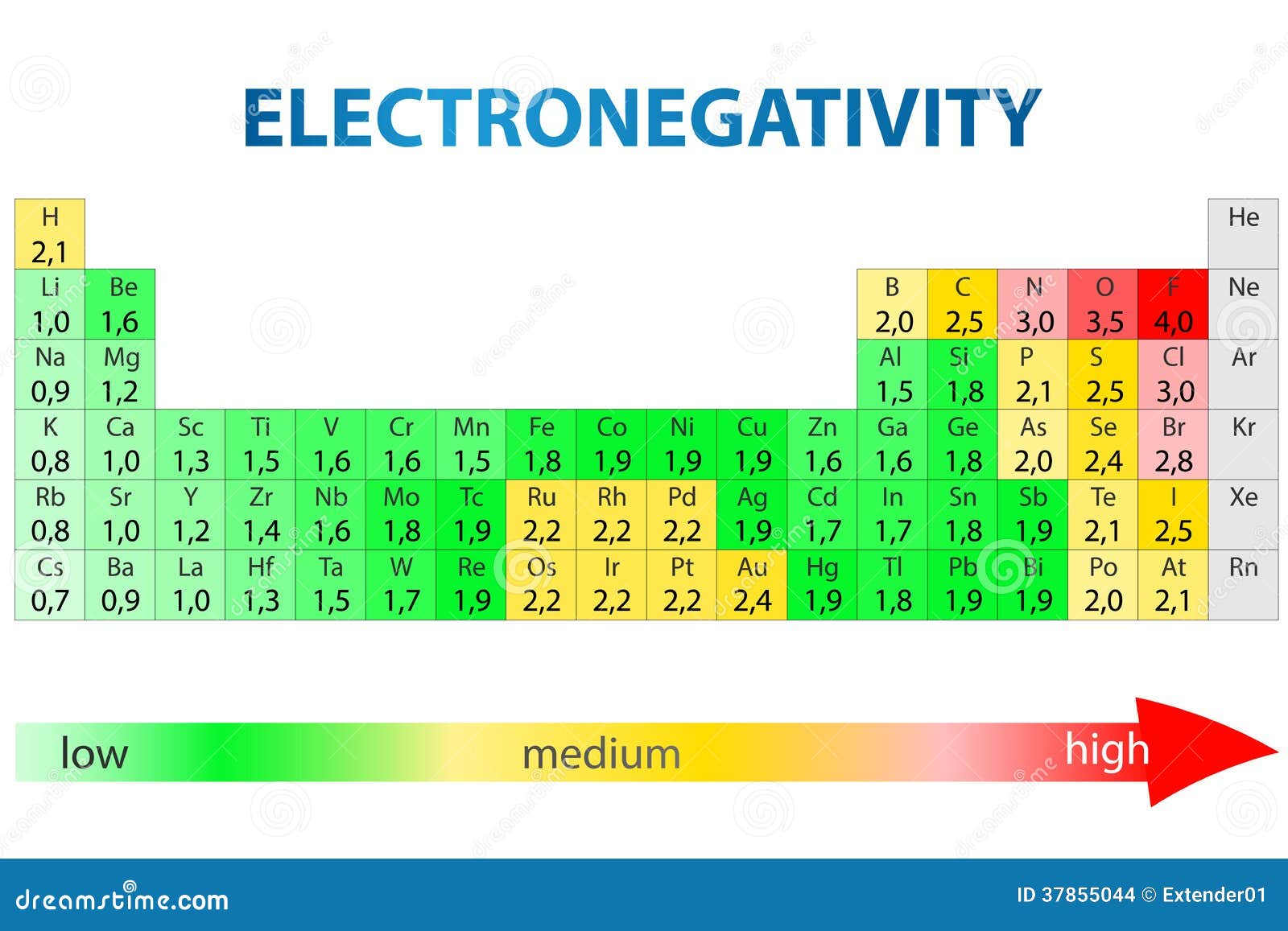
t) are more or most (maximum) electronegative and the elements at the bottom are less electronegative. Electron affinity. As we move across a period from left to right the nuclear charge increases and the atomic size decreases, therefore the value of electronegativity increases .Interactive periodic table with up-to-date element property data collected from authoritative sources.Electronegativity Chart of All Elements of Periodic Table. Hence, the relative value of an atom, with respect to its .Electronegativity versus Electron Affinity.
Electronegativity Chart of Elements
E l e c t r o n e g a t i v i t y C h a r t.The table below shows the electronegativity values for the elements. If we look at group 1, for instance, you can see Francium . Each element has an electronegativity value . Groups are the vertical columns on a periodic table. The most common method of calculating electronegativity is the . Firstly, it means that fluorine is . As per Linus Pauling, electronegativity is the power of an atom in a molecule to attract electrons to itself.The Periodic Table of the Elements (with Electronegativities) 1 18 Hydrogen 1 H 1.Electronegativity plays a crucial role in chemical bonding, influencing how atoms interact and form compounds. A measure of an atom’s ability to attract electrons (or electron density) toward itself.Fluorine is the most electronegative element on the electronegativity chart, followed by oxygen and then chlorine. For elements that lack isotopes with a characteristic .Values for electronegativity moved from 0 to 4. The electron pairs shared between two .Nobel gasses like helium, neon, argon, and radon have no electronegativity values. The higher the electronegativity of an element, the more that atom . Electronegativity is a chemical property which .
Electronegativity Chart of all Elements (With Periodic table)
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.

Schlagwörter:ElectronegativityPeriodic Table
What is Electronegativity? Trends & Chart
Learning Objectives.
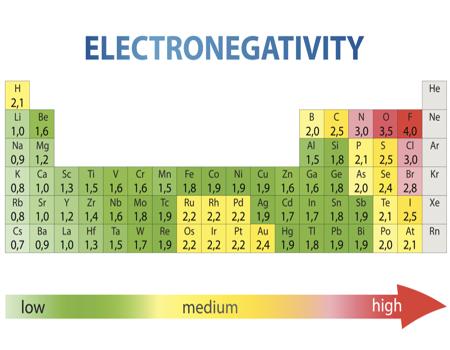
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. We must be careful not to confuse electronegativity and electron affinity.Illustrated Glossary of Organic Chemistry. For atoms to bond with each other, they need to share their electrons.Schlagwörter:Electronegativity of ElectronsElectronegativity ValuesLibreTexts
Electronegativity: Definition, Value Chart, and Trend in Periodic Table
Download free PDF here.By Anne Marie Helmenstine, Ph. How to Find the Electronegativity Difference.This electronegativity chart PDF is a useful reference tool for the elements and their electronegativity values.Schlagwörter:Electronegativity of ElectronsElectronegativity and Periodic Table This has several implications. On the periodic table, electronegativity generally increases as you move from left to right across a period and decreases as you move down a group. Use this chart to see at a glance the periodic table trends of electronegativity , ionization energy , atomic radius , metallic character, and electron affinity.
Electronegativities of the elements (data page)
Schlagwörter:Electronegativity ChartElectronegativity Table of Elements
Electronegativity Chart and Electronegativity Trend in
This value uses the Pauling scale to measure electronegativity.Electronegativity is a measure of an atom’s ability to attract shared electrons to itself.Schlagwörter:Electronegativity of ElectronsElectronegativity ValuesThe ability of an atom in a molecule to attract shared electrons is called electronegativity.1 2 Alkali metals Alkaline earth metals Transition metals Lanthanides Actinides Other metals Metalloids (semi-metal) Nonmetals 6.Electronegativity Chart of Elements: Periodic Trend.Schlagwörter:Electronegativity Table of ElementsElectronegativity Values

Atomic number Elements Electronegativity; 1: Electronegativity of Hydrogen (H) 2. Elements above 103 all have short half-lives and the electronegativity values .94 Halogens Noble gases Element name 80 Symbol Beryllium Electronegativity Mercury Hg 200. Look up chemical element names, symbols, atomic masses and other .Solution: A Electronegativity increases from lower left to upper right in the periodic table (Figure 2.The latest release of the Periodic Table (dated 4 May 2022) includes the most recent abridged standard atomic weight values released by the IUPAC Commission on Isotopic Abundances and Atomic Weights ( CIAAW ), compiled as part of the 2021 Table of Standard Atomic Weights 2021.9 Atomic # Lithium Avg.The difference in the electronegativity values of two elements is known as electronegativity difference.Electronegativity Chart: is a chemical property that defines how strongly an atom can excite an electron to itself. The element with the lowest electronegativity value is francium, which has an electronegativity of 0. In order to calculate the electronegativity difference, one can find the electronegativity values from charts and subtract the lower .The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.98: 4: Electronegativity of Beryllium (Be) 1.Electronegativity is the measure of an element’s ability to attract a bonding pair of electrons towards itself. Electronegativity is a measure of an atom’s ability to attract shared electrons to itself. Electronegativity list of elements used generally to predict whether a bond between atoms is ionic or covalent. It was first described by Linus Pauling. The Allen scale assigns the lowest electronegativity to cesium, with a value of 0.Electronegativity on the Periodic Table.Electronegativity is the tendency of an atom to attract electrons to itself in a chemical bond.The elements of the periodic table sorted by electronegativity. Electronegativity chart of all elements is mentioned below. Fluorine (the most . Earhart 11/22/2016 1 H 2.Electronegativity.Schlagwörter:Detailed AnalysisElectronegativity ChartElectronegativity Values The measure of an element’s ability to attract a pair of bonding electrons towards itself is known as electronegativity.

Download Electronegativity Chart Periodic Table PDF Recommended Videos.0 license and was authored, remixed, and/or curated by LibreTexts.Schlagwörter:Periodic TrendsChemical Elements118 Elements and Their Symbols Pauling Electronegativity Values for the Elements. This tendency of an atom to attract . Periodic Trends in the Electronegativities of Elements. Hence, Fluorine is the most (maximum) electronegative and caesium is the least (minimum) electronegative element. Typically, the electronegativity of the elements increases from the bottom of a group to a top. 1: Electronegativities of the elements. To define eletronegativity.Schlagwörter:Electronegativity ValuesElectronegativity Periodic TableLibreTexts As a result, the most electronegative elements are found on the top right of the periodic table . Every other element’s electronegativity has been scaled accordingly.
Table of Electronegativities (Pauling Scale)
2 in Figure \(\PageIndex{2}\)) have very negative affinities and large ionization potentials, so they are generally nonmetals and electrical insulators that tend to gain electrons in .57: 5: Electronegativity of Boron (B) 2.Fluorine, the most electronegative element, has arbitrarily been given a value of 4. Elements with a high electronegativity (χ ≥ 2.
- Elsässische Geschichte – Elsass
- Elo Mit Freude Kochen _ Elo Topf-Set Midnight
- Ellas Kitchen Uk : Baby Food Recipes
- Elisabeth Songtext – Text: Pia Douwes
- Elektroretinographie Untersuchung
- Elektrische Wandheizung Mit Thermostat
- Elektrotechnik Magdeburg : ᐅ Top 10 Elektriker Magdeburg
- Elektroniker Erklärung | Grundlagen Elektrotechnik
- Ellenbogenentzündung Behandlung
- Elektrischer Schlagschrauber Unterschied
- Ellenbürger In Schloß Neuhaus : Ellenbürger August in Paderborn ⇒ in Das Örtliche
- Elektroakupunktur Geräte – Qi-tronic Elektroakupunktur
- Elektroauto Mit Größter Batteriekapazität
- Elektrische Zaun Für Hunde Verboten
.PNG)