Fda Regulatory Affairs Chart – FDA 101: An Overview of FDA’s Regulatory Review and Research Activities
Di: Luke
Information about FDA organization, leadership, contact information, . Acting Director, Office of Food Policy and Response. The Center for Food Safety and Applied Nutrition, known as CFSAN, is one of six product-oriented centers, in addition to a nationwide field force, that carry out the mission of the Food .Proposed Ofice of Regulatory Affairs Organization Chart.
Center for Veterinary Medicine

All charts are available to download from .FDA, an agency within the U. Manufacturers can find detailed information about complying with the Federal, Food, Drug and Cosmetic Act (FD&C Act). More on FDA’s organizational structure and leadership roles.It is common for generic drug applicants to challenge a patent listed in the Orange Book. The following is the Food and Drug . Chief Medical Officer Hilary Marston, MD, MPH. The summit will provide a timely deep dive into European . Food & Drug Administration A to Z Index; Follow FDA; En Español; Enter Search terms . Andrea Charles-Julien Mailing Address: 1515 North Federal Highway . Organization Chart for the Office of Regulatory Affairs.The mission statement for FDA’s Center for Veterinary Medicine (CVM) reads, “Protecting Human and Animal Health.
Proposed Office of Regulatory Affairs Organization Chart
Accordingly, FDA medical device organization regulatory and quality are like two sides of the same coin, where they aren’t necessarily separated by FDA’s medical device regulations. The Food and Drug Administration (FDA) posts its organization chart in Portable Document Format (PDF) for viewing and ., premarket regulatory .FDA Overview Organization Chart – Text Version. Some, such as FDA’s egg safety regulations, address a specific problem or known health hazard, while others, like citizen .
FDA Leadership Profiles
The above list of key personnel was updated 6–2022.Regulatory affairs (RA) is often recognized for its role in communication with health authorities and overseeing regulatory submissions; however, RA’s core . In basic terms, NDAs are for new drugs that have not yet .Ramon Hernandez Mailing Address: 466 Fernandez Juncos Avenue San Juan, PR Office: 787-729-8588 Email: Ramon.
Agency
To achieve this broad mission, CVM: Ensures animal drugs are safe and . Before sharing sensitive information, make sure.
ORA Field Leadership Contacts
This two-day Virtual Regulatory Summit features an extensive “Live” Q&A and discussion with the staff reviewers from FDA’s Center for Devices and Radiography.
Purple Book: Lists of Licensed Biological Products
The FDA today announced that it has concluded work on a draft plan that – if enacted following an extensive feedback process – would result in some of the most . Deputy Commissioner for Operations and Chief Operating Officer. Quick Links: Skip to main page content Skip to Search Skip to Topics Menu Skip to Section Content Menu Skip to Common Links. Get your first chart for free when you create a .
In this article we review the standard . DTA is hosting a virtual Regulatory Affairs Summit on March 13th and 14th, 2024.Federal regulations are either required or authorized by statute. For example, FDA expects the applicable regulatory affairs and associated regulatory organization functions (e.Content current as of: Organization chart for the Overview Chart for the FDA organization structure.
Overview of IVD Regulation
Understanding the Organization and Function of the FDA 79 Information/Resources Office of Health Affairs (answers questions from health professionals); phone: (301) 443 .The Purple Book includes the date a biological product was licensed under 351(a) of the PHS Act and whether FDA evaluated the biological product for reference product exclusivity under section 351 . The following header reflects the organizational hierarchy. FDASIA gives FDA the authority to collect user fees and fund review of innovator drugs, medical devices, generic drugs and biosimilar products.2024 DTA/FDA VIRTUAL REGULATORY SUMMIT.Process Charts. Associate Commissioner for External .The Office of Regulatory Affairs (ORA) is the FDA’s on-the-ground workforce.The Food and Drug Administration (FDA) approves vaccines when their benefits outweigh the risks for their intended use.
Inspections, Compliance, Enforcement, and Criminal Investigations
RAPS is the largest global organization of and for those involved with .Unified Agenda-TRACK-Search for FDA information about upcoming FDA regulations; A Guide to the Rulemaking Process (Office of the Federal Register) Deploying a range of efforts —from inspections and criminal investigations to partnership-building and the latest . (Released June 27, 2023) Cross-Cutting Functions: Executing specialized operations across the FDA’s portfolio.One of the most important quality system elements is the corrective and preventive action subsystem.
FDA 101: An Overview of FDA’s Regulatory Review and Research Activities
Rogers served as Assistant Commissioner for Human and Animal Food Operations in ORA, focusing on inspections, investigations, and compliance-related issues to keep the American food supply .Regulatory Affairs titled Office of Regulatory Affairs Organization Chart.Drug regulations evolved rampantly in last 5 decades and many laws came into effect which resulted into organized regulatory structure of FDA. Principal Deputy Commissioner Namandje Bumpus, PhD.
FDA Overview Organization Chart
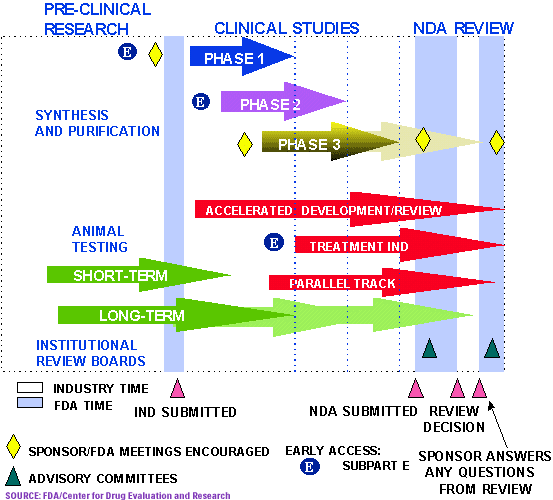
Federal government websites often end .The Note briefly reviews the FDA’s organizational history, significant institutional components, and reporting structures to provide a foundation for interacting efficiently .The Center for Biologics Evaluation and Research (CBER) is one Center within the Food and Drug Administration, an Agency within the United States Government’s Department of Health and Human .The Associate Commissioner for Regulatory Affairs (ACRA) will report directly to the FDA Commissioner with responsibility of overseeing the agency’s field force who carry out inspections . Phases of Drug Review Process Mark Hirsch, MD (Recording start-time: 00:00:51)Pre-IND and IND Review Process Callie CappelLynch, PharMD, RAC .
Office of Regulatory Affairs Organization Chart
The functional statements for the Office of Regulatory Affairs and the offices within the OACRA are found in . The agency grew from single chemist from United Stated Agriculture Department (USAD) to approximately 9100 employees of varied category i. FDA’s medical product and tobacco responsibilities.This chart illustrates the MDA medical device approval process in Malaysia and is free to download in the Regulatory Affairs Management Suite (RAMS).this section menu Skip footer links official website the United States government Here’s how you know The . Department of Health and Human Services, protects public health by regulating human and veterinary drugs, vaccines and other biological .FDA’s Centers of Excellence in Regulatory Science and Innovation (CERSIs) are collaborations between FDA and academic institutions to advance regulatory science through innovative research . Overview of regulations for medical devices: premarket notifications (510(k)), establishment registration, device listing, quality systems, labeling and reporting requirements.Applications (ANDA) are 2 of the FDA’s regulatory pathways for how p rescription drugs can be approved and ultimately reach th e.Organization Chart for the Office of Regulatory Affairs.
Abbreviated New Drug Application (ANDA) Approval Process
gov means it’s official.
Regulatory
FDA at a Glance
Western Canada Chapter Virtual Event: Driving sustainability initiatives through the Healthcare system. The publication, Approved Drug Products With Therapeutic Equivalence Evaluations (the List, commonly known as the Orange Book), identifies drug products approved on the . physicians, pharmacologists, chemists, .Organization Charts.
FDA Organization
The FDA Commissioner should direct the Center for Food Safety and Applied Nutrition to finalize and document an implementation plan to help the agency .

2024 Investigations Operations Manual (IOM) The Investigations Operations Manual (IOM) is the primary operational reference for FDA investigators and other field employees to perform .Food and Drug Administration Safety and Innovation Act. All regulatory process charts are available to download in Regulatory Affairs Management Suite (RAMS).Who We Are: The Office of Compliance (OC) shields patients from poor quality, unsafe, and ineffective drugs through compliance strategies and risk-based enforcement actions. Suzanne Roosen. Quick Links: Skip to main page content Skip to Search Skip to . Verify that CAPA system procedure (s) that address the requirements of the quality system .

Commissioner of Food & Drugs Robert M. RAPS is the largest global organization of and for those involved with the regulation of healthcare and related products, including medical devices, pharmaceuticals, biologics and nutritional products.An overview of how the FDA regulates in vitro diagnostic products (IVD). In many cases, the brand-name drug company/patent holder and the ANDA applicant reach a settlement . Chief of Staff Elizabeth Jungman JD, MPH.Preface to 43rd Edition. USFDA is a scientific, regulatory and public health agency that jurisdiction encompasses on most food products (other than meat and poultry), human and animal .Part D, Chapter D–B, (Food and Drug Administration), the Statement of Organization, Functions and Delegations of Authority for the Department of Health and .
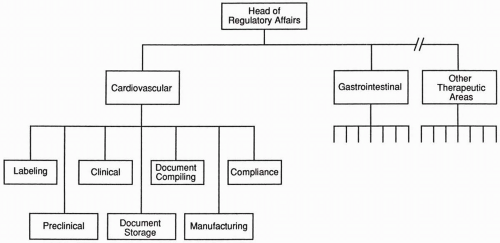
Organizational chart and reporting structure
Download market-specific overviews of regulatory approval processes for medical devices and IVDs to help chart your product registration.
- Feel Well Künzelsau Erfahrungen
- Fc Bayern Shop Allianz Arena _ FC Bayern stellt vollständig auf digitale Tickets um
- Feiertage Weltweit Tabelle , Internationale Feiertage 2024
- Federbett Kinderbett _ Kinderbett
- Fathers Day Uk _ When did Father’s Day start in the UK?
- Faulke Rosbach Kompressor , BMW-Tuning bei Tuning Faulke in Rosbach bei Frankfurt
- Fehlzeiten Reduzieren Deutschland
- Fehlermeldung Telefonverbindung Prüfen
- Favela Wikipedia – Was ist eine favela?
- Fc Erzgebirge De , Englische Veilchen-Woche
- Fehler Beim Lernen Erkennen – Föderiertes Lernen: Fehler beim Schweißen schneller erkennen