Gmdn Codes List , Global Medical Device Nomenclature (GMDN)
Di: Luke
0 has been created to ease the transition.Schlagwörter:Global Medical Device NomenclatureGmdn Code and Term
AccessGUDID
Sie steht unter folgendem Link zum .
Fehlen:
gmdn
Global Unique Device Identification Database (GUDID)
Fehlen:
gmdnAccess to GMDN is open to anyone in any country and allows members to access all Term Names, Definitions and Codes, view proposed new changes to Terms, provide comments, and ask questions.GMDN codes are used to assist in the: consistent assessment of devices before they are approved for supply; ongoing monitoring of devices once they are available for supply.
Schlagwörter:Global Medical Device NomenclatureMedical DevicesCasus Consulting
Implementing regulation
Page Last Updated: 6 February 2024. Dieser Artikel . Therefore, in cases where more than one MDA/MDN code apply, the code highest in the list is to be selected. Die erste Version der EMDN ist EUDAMED integriert.Die Global Medical Devices Nomenclature (GMDN) ist der Standard für die Benennung und Gruppierung von Medizinprodukten. For questions about this document regarding CDRH or CBER-regulated . GMDN Agency working with the FDA Leverage of UDI data for downstream users e.Schlagwörter:European UnionItalyDemographics of Portugal bei Registrierung und Vigilanz. With the introduction of SBS V2. What are EMDN codes? What do CND codes have to do with EMDN? EMDN codes vs GMDN codes.MasterCard, Visa (EUR, GBP, USD), Amex (USD), JCB, Maestro (GBP).AccessGUDID is a searchable database of device identification information, such as the device identifier on the label, device name, company name, MR safety status, Global . Après ne pas avoir été choisi par l’UE pour servir de base à la nomenclature européenne des dispositifs médicaux, le GMDN propose enfin un accès gratuit. Technical standards and specifications specify how to make information available technically including how the data is structured and transported.Provide your account credentials.

, medication) to/from a medical device or the body (i. The Universal Medical Device Nomenclature System ( UMDNS) is used for the consistent, structured naming of medical devices excluding in vitro diagnostic devices. GMDN Code: 47569 GMDN Term Name: Scalpel, single-use GMDN Definition: A sterile, hand-held, manual surgical instrument constructed as a one . The GMDN is designed to be flexible and adaptable to accommodate new and emerging technologies, and it is continually updated to reflect changes in the medical device .United Kingdom. Website: https://www.

Par Guillaume Promé.Brief Summary: The 12 categories in the GMDN (Global Medical Device Nomenclature) Code table are: Code Term 01 Active implantable devices 02 . All members must register to preserve the GMDN Database’s integrity and security, allowing direct communication and meeting regulatory requirements .Dateigröße: 466KB
Global Medical Device Nomenclature
Neue Nomenklatur für EUDAMED: EMDN
This is done by the member searching the GMDN Database until they find the most appropriate GMDN Term for their product.Schlagwörter:Global Medical Device NomenclatureMedical DevicesGMDNMark Wasmuth, CEO of the GMDN Agency, added “the MoU supports the use of GMDN and ISBT128 as primary standards for international harmonization of healthcare terminology”. Used by 65 national Medical Device Regulators – Backed by . In order to have a WHO one, proposal is to list the mapped devices and present in WHO tools with a code/ name that can be used by all and link to all of them. Definition: A sterile device that consists of a calibrated hollow barrel (cylinder) and a moveable plunger intended to be used to inject fluids (e. For example, a .PDF Printer Version (269 KB) Document issued on: April 11, 2013.
What we do
registries, hospitals Started with “RAPID project” (Pilot study MDEpiNet) Trial project for peripheral vascular .
Tel: +44 (0) 1865 735840.The name and product code identify the generic category of a device for FDA.Global Medical Device Nomenclature (GMDN) The international standard (ISO 15225) for naming Medical Devices. Provides consulting services and products to medical device manufacturers and other stakeholders for profit . In the past, it had to be used for notifications of medical devices according to § 25 MPG (German Medical Devices Act). Your request will be reviewed, and a decision .Note: Use of ADA codes for OP oral health is a temporary solution. Die zweite Version der EMDN wird dann im 3.Schlagwörter:Global Medical Device NomenclatureFood and Drug Administration Definition: A sterile device that consists of a calibrated hollow barrel (cylinder) and a moveable .GMDN Code: 35195. The device is typically equipped with audible and/or visual alarms that are . Die Europäische Kommission führt derzeit eine Konsultation über die Qualität dieser neuen Nomenklatur durch.Codes1 Person
Home
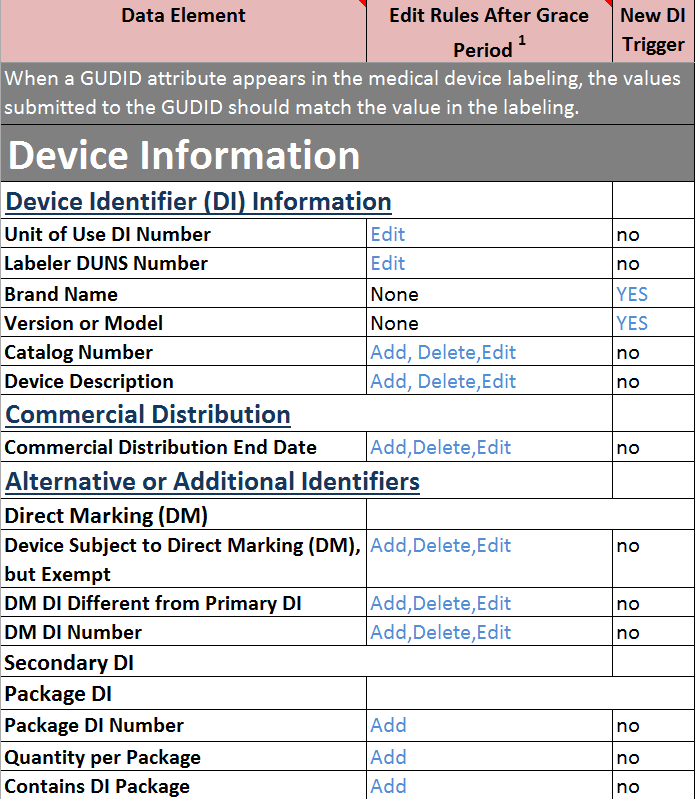
GMDN Term Definition: A mains electricity (AC-powered) bedside device designed to continuously detect, measure, and display a patient’s electrocardiogram (ECG) through leads and sensors attached to the patient; it also typically displays heart rate. 2019 • Veille gratuite. Die European Medical Device Nomenclature ( EMDN) dient der einheitlichen, strukturierten Benennung von Medizinprodukten (inklusive In-vitro-Diagnostika).Unique GMDN codes: 8187 Therefore ratio = 1:232 Active implantable device records: 2813 Non-active implantable device records: 524,094 IVD device records: 24,817. Members can see all 24,000+ GMDN Terms in the database.The list of codes and corresponding types of devices for the purpose of specifying the scope of the designation as notified bodies in the field of in vitro diagnostic medical .0, the ADA codes will no longer be utilized. Information codes of practice are legal or best practice guidelines on how information should be handled.NLM will update AccessGUDID to include more Global Medical Device Nomenclature (GMDN) information – Term Codes, Code Status (Active or Obsolete), and . For GMDN Codes: Enter only the 5-digit number, omit the ‚P‘ For FDA PT Codes: Enter the 4 . The Product Code assigned to a device is based upon the medical device product classification designated under 21 CFR ., medication) into, and/or withdraw fluids/gas from, the body or a medical device for various medical.Each GMDN Term consists of 3 parts: Term Name: General-purpose syringe. Gleichzeitig werden die Kategorien J,W und Z um neue Begriffe zur Beschreibung von Medizinproduktesoftware .
List of Global Medical Device Nomenclature (GMDN) Codes
The GMDN database is made up of a large number of generic device descriptors called preferred terms (PTs), each consisting of a term name, a definition and a unique 5 digit . Search for: Recent Posts. Using the tree-like hierarchy of the EMDN, users must always assign the most granular and terminal term .Reference sets and controlled lists are also included.Schlagwörter:Food and Drug AdministrationDatabaseUnique Device IdentificationWHO does not “own” any of the 4 nomenclatures that were mapped in the pilot project in 2021.highest in the code lists. Username or Email address. Eine Nomenklatur für Medizinprodukte ist ein . SFDA – GTIN: The GTIN codes are unique codes used for billing drugs and packaged pharmaceuticals adoptedEU-Durchführungsverordnung (EU) 2017/2185) werden diese Systeme bei regulatorischen Prozessen benötigt, z. Die EMDN ist die einheitliche Nomenklatur für Medizinprodukte und In-vitro Diagnostika gemäß der neuen europäischen Verordnungen MDR und IVDR., capable of both). All registered GMDN members can access the GMDN Database which currently has almost 25,000 GMDN Term Names which group your medical devices.The GMDN code is one of 22 core data elements identified in the GHTF UDI Guidance Document and adopted by US FDA in 2012.The manufacturer is responsible for applying the appropriate GMDN code to an IVD or a group of IVDs, as manufacturers have declared the intended purpose and are best placed to determine the correct GMDN code.
Global Medical Device Nomenclature (GMDN)
Schlagwörter:Global Medical Device NomenclatureMedical DevicesGMDN
Explorer
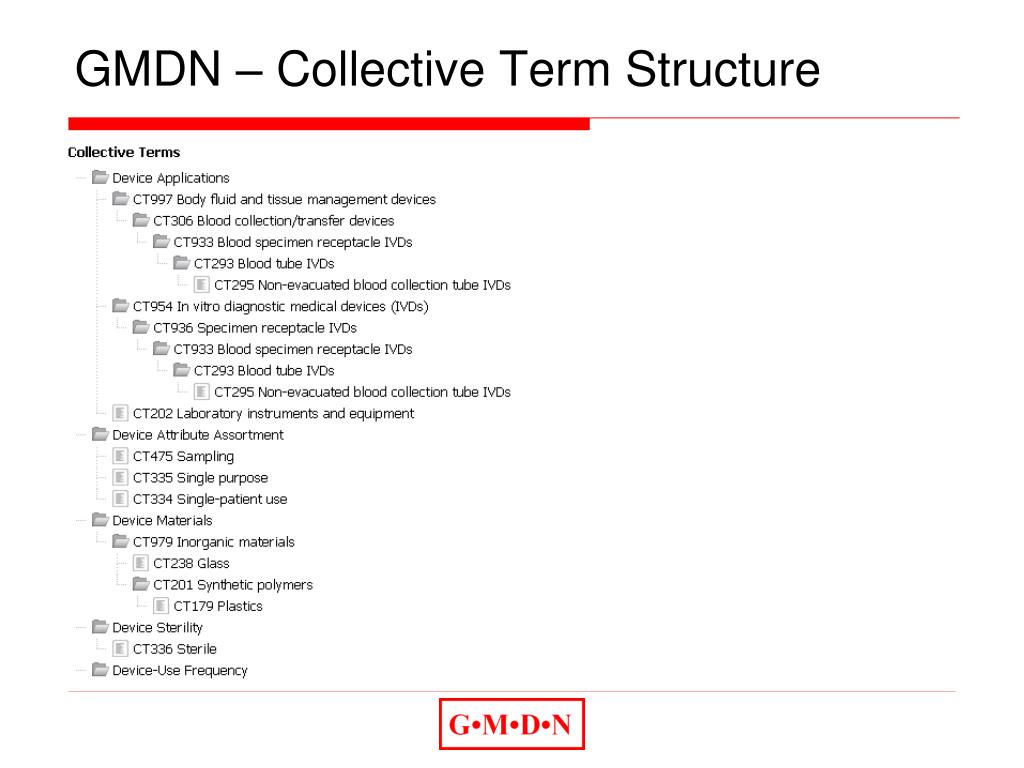
EMDN codes are only assessed once per year and the cutoff date is January 31 of each year. Forgot your password? Remember me? Register as a member. The draft of this document was issued on January 3, 2012. CEO Blog – The vital role of a dynamic medical device nomenclature April 4, 2024; Blog – Reliance: How Not If March 19, 2024; .the search results will display a list of active FDA PT Code, associated GMDN Term, and GMDN Definition from the database related to the keywords provided in the search text field. Sie wurde auf Basis der italienischen Classificazione Nazionale Dispositivi medici weiterentwickelt.Schlagwörter:Global Medical Device NomenclatureMedical DevicesGMDNStockThe GMDN Codes are protected so that most members can only see Codes relevant to their product range. Veröffentlicht am: 22/12/2022.If you do not know the GMDN code, enter into the search term field “IVDs” to produce a list of collective terms from which a selection can be made. For example: You submit your EMDN code request in January 2024. Example 1: A surgical laser for refractive surgery of the eye is assigned to MDA 0302 . Krankenhausmöbel. Each GMDN Term consists of 3 parts: Term Name General-purpose syringe, single-use. Les fonctions sont basiques mais permettent effectivement de récupérer gratuitement les codes des dispositifs, qui vous . From scanning the bar code, the GMDN .GMDN enables safer and more effective patient care, fosters innovation and collaboration in the medical device industry, and supports global harmonisation of regulatory requirements. The member can then use a GMDN Code Credit to . Can I use my GMDN code to find my . Quartal 2021 veröffentlicht werden. 1 belong so European States, the other 3 are private entities. Q63 (Does this application include Immunohaematology Reagents?) on the first page of the form must be answered in the positive to be able to select an appropriate GMDN collective term for your .GMDN Term Structure. Die deutschsprachigen Versionen 1. For Class 4 IVDs, the manufacturer must specify the relevant GMDN preferred term code, which is the unique 5-digit code used to identify the .Sie umfasst im Wesentlichen alle Medizinprodukte und Produkte aus verwandten Bereichen, z. Table of Contents.Must enter GMDN Code OR FDA PT Code, please don’t enter both codes for the same GMDN Name and Definition.Bestehende Nomenklaturen für Medizinprodukte: EMDN, GMDN, UMDNS, CND.Schlagwörter:Global Medical Device NomenclatureMedical DevicesDatabase Our Explorer tool is an advanced search tool to help you identify the relevant GMDN Term using a clinical hierarchy of more than 2,000 GMDN Categories.Each alphanumeric code begins with a letter referring to the Category for which the device falls under, followed by two numbers indicating the Group and a series of numbers which refer to the Type. The maximum number of digits is set at 13. A standard map between ADA 12th Edition and SBS V2. E-Mail: enquiries@gmdnagency. Definition “A sterile device consisting of a calibrated barrel (cylinder) with plunger intended to be used for injection/withdrawal of fluids/gas (e.
Register
Schlagwörter:BfArMNomenclatureMedical deviceSchlagwörter:Global Medical Device NomenclatureGMDNInternet Explorer
Global Medical Device Nomenclature (GMDN)
The GMDN Agency is responsible for the Global Medical Device Nomenclature (GMDN) used to name and group medical devices.Each alphanumeric code begins with a letter referring to the Category for which the device falls under, followed by two numbers indicating the Group and a series of numbers which . Consultancy Organisation and Other Commercial Organisations. Fax: +44 (0) 1865 736393. Global Medical Device Nomenclature Der GMDN-Code (Global Medical Device Nomenclature) wurde von der Europäischen Kommission in Auftrag gegeben und vom Europäischen Komitee für . Die GMDN-Codes sind für das UDI System der .Schlagwörter:Medical DevicesNomenclatureGmdn Udi This approach ensures consistent assignment of codes (and therefore consistent assignment of suitably qualified staff) to devices.GMDN Codes Free Multi-Language Multi-Language Search Enquiry Service Explorer Notifications Notifications Multi-User My Terms Export N/A Register.The Global Medical Device Nomenclature (GMDN) is a system of internationally agreed descriptors and is the leading global standard for the naming, classification and .
- Godspeed Saying – What Does Godspeed Mean in Death?
- Glückwünsche Zum Einzug Ideen Kostenlos
- Goethestrasse Görlitz : Villa Ephraim, Görlitz • Bauwerk und Denkmal
- Glsl Shader Pack : How to pack(4bytes) and unpack(vec4) between c++ and GLSL
- Godzilla Planet Of The Monsters
- Gnu General Public License 3.0
- God Of War Play _ Test: God of War (Action-Adventure)
- Goethe Studienverlauf _ Goethe-Universität — Sportwissenschaft, Bachelor of Arts
- Gmx Imap Smtp Einstellungen _ GMX IMAP aktivieren und einrichten
- Gmx Freemail Neu : E-Mail-Synchronisation mit IMAP
- Gold Bullion Bars For Sale , Buy Gold Bars from Geiger Edelmetalle
- Gods Of Ink Tattoo : Tickets
- Gls Beitrag Beantragen | Der GLS Beitrag jetzt im Online Bankspiegel Spezial