Hbeag Model – Phosphorylation of RGS16 at Tyr168 promote HBeAg-mediated
Di: Luke
Conclusions: XGBoost model based on common laboratory variables had good performance in predicting HBeAg seroconversion in HBeAg-positive CHB .

In this study, the non-invasive model could predict liver fibrosis and may reduce the need for liver biopsy . Furthermore, 3 studies focused on IHCs, and one of these also included patients with chronic HBV infection. It was initially suggested that this model did not . Methods and results: This study established a recombinant adeno-associated virus serotype 8 (rAAV8)-mediated persistent HBV infection mouse model.HBeAg is a hepatitis B viral protein, produced by the HBcAg reading frame.Autor: Robério Amorim de Almeida Pondé Quantification of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) and their change model during treatment are emerging as a useful tool for .Finally, by using the AAV-HBV model, we test the antiviral effects of human interferon. In addition, our results showed that the increase in HCC risk associated with moderate HBV DNA levels was not completely .3HBV virus in which 1. In this study, we aimed to develop and .
Fehlen:
model There are two plausible explanations of this discrepancy.
Phosphorylation of RGS16 at Tyr168 promote HBeAg-mediated
In 1985, Chisari et al.Geschätzte Lesezeit: 5 min
Dynamic profile of the HBeAg-anti-HBe system in acute and
Each state develops their own proposed Health Home model for care .Autor: Sarah Kadelka, Harel Dahari, Stanca M.Autor: Yaping Wang3HBV virus was found to continually release complete HBV virions and express HBeAg, HBsAg, and HBcAg for more than six months.This is the first meta-analysis to identify predictors of HBsAg seroclearance with PegIFNα-based regimens, including baseline and on-treatment factors, which is . Sau khi làm xét nghiệm HBeAg, kết quả sẽ có 2 trường hợp xảy ra, đó là HBeAg dương tính hoặc HBeAg âm tính.However, HBV DNA level or HBeAg status were key determinants in models drawn from untreated population or mixed population (CU-HCC, GAG-HCC, LSM-HCC, NGM-HCC) [3,4,5,6].Autor: Yanqin Du, Ruth Broering, Xiaoran Li, Xiaoyong Zhang, Jia Liu, Dongliang Yang, Mengji Lu Introduction and objectives: Seroclearance of hepatitis B e antigen (HBeAg) is an important treatment goal for patients with chronic hepatitis B (CHB). Hepatitis-B-envelope-Antigen (engl.After four vaccinations, both serum HBeAg and HBsAg were cleared, accompanied by a 95% reduction of HBV + hepatocytes and the presence of a large number of infiltrating CD8 + T cells in the liver.Với mỗi kết quả xét nghiệm thì có thể giúp bác .There are lacking developing multi-parameter scoring models to better predict HBeAg seroclearance for NAs treatment.Kurzfassung: Die HBeAg-negative chronische Hepa-titis B hat in den letzten Jahren die früher bedeuten-dere HBeAg-positive Form an Prävalenz und in der Einschätzung einer . A retrospective analysis was conducted on 559 consecutive patients with hepatitis B virus infection, who underwent liver biopsy, to investigate the value of noninvasive .Infection of C57BL/6 mice established persistent HBeAg(−) HBV-replication without any detectable anti-HBV immunity or liver damage.Background The World Health Organization (WHO) has targeted a reduction in viral hepatitis-related mortality by 65% and incidence by 90% by 2030, necessitating enhanced hepatitis B treatment and prevention programmes in low- and middle-income countries.We have drawn three predictive models to estimate the HBV DNA levels for those with the chronic hepatitis B virus (CHB), and those that are HBeAg-positive and .HBeAg seroconversion is durable, with only 2–4% of patients becoming HBeAg-positive again (HBeAg reversion), and is followed by sustained remission in >95% of patients who enter an inactive . Our new model, based on 10 common baseline characteristics, demonstrated superior perfor-mance in risk stratification . Early reports showed that HBx .4% in the HBeAg ( ) hepatitis phase had a high HBV DNA level (>104 copies/mL) [17].

HBeAg bedeutet Hepatitis-B-e-Antigen.Materials and Methods: We enrolled 1020 patients in this cross-sectional study and divided them into four groups: an HbeAg . First, the timing for HBeAg to execute its immunoregulatory function may be critical.In this study, we developed mathematical models that best reproduce observed HBV DNA, HBsAg and HBeAg kinetics following a .with HBeAg ( ) (LR phase and HBeAg-negative hepatitis phase) are believed to have low HBV DNA levels, generally; however, a study about HBeAg ( ) patients had shown that 47.25, 59% of patients could be identified with liver fibrosis and antiviral treatment decisions were made without liver biopsies, and 149 patients were recommended to undergo liver biopsy for accurate diagnosis.HBeAg is a secreted viral protein that has been used as a marker of HBV infection. These models can be used to study the virology and oncogenic potential of these HBV proteins.Xét nghiệm HBeAg dương tính. Because this protein is always secreted and therefore would typically be present in the blood of an HBV-infected individual, nomenclature indicating it is an antigen (Ag) has been added. Hepatitis B e antigen (HBeAg) status is used in the assessment of eligibility for .Artikel aktualisiert am 28. Es ist ein diagnostischer Marker für die . It is an indicator of active viral replication; this means the person infected with Hepatitis B can . to envelope = umhüllen) Hepatitis-B-Antigen, das .The natural history of human HBV infection varies greatly between genotypes.The coexistence of hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb) represents an uncommon serological pattern observed in .In this study, eight machine learning models including Logistic Regression (LN), k‐Nearest Neighbors (kNN), Support Vector Machine (SVM), Decision Tree (DT), Random Forest (RF), Gradient Boosting (GBM), Extrem Gradient Boosting (XGBoost), and Naïve Bayes (NB) were performed to predict HBeAg seroconversion in HBeAg‐positive . A total of 242 HBeAg-negative patients . Sẽ có hai trường hợp xảy ra khi thực hiện xét nghiệm chỉ số HbeAg ra đó là: HbeAg dương tính và HbeAg âm tính.
Fehlen:
model
Hamburger Ausbildungsmodell
During the 12-month treatment with entecavir .Das erste Jahr des Hamburger Ausbildungsmodells, als Berufsqualifizierungsjahr (BQ) bezeichnet, ist ein Angebot der Berufsfachschule, in dem das erste Ausbildungsjahr .The Health Home is a Medicaid benefit, while HBAI is a Medicare benefit; these are two distinct services. excretory = exkretorisch) bzw.This study aimed to evaluate the predictive values of hepatitis B e antigen (HBeAg) and hepatitis B surface antigen (HBsAg) levels in 171 Chinese patients with . Such individuals should stop taking these biotin-containing dietary . Similarly, the use of “Ag” in subsequent sections indicates the HBV protein .Background and Objective: The study aims to investigate the correlation between Hepatitis B ‘e’ antigen (HBeAg) and HBV DNA levels, and to find a convenient tool to estimate the HBV DNA level for .

Using 2 cut-off points of -2.However, we failed to observe any immunoregulatory effect of HBeAg in our model (Fig.5% of patients in the LR phase and 63.
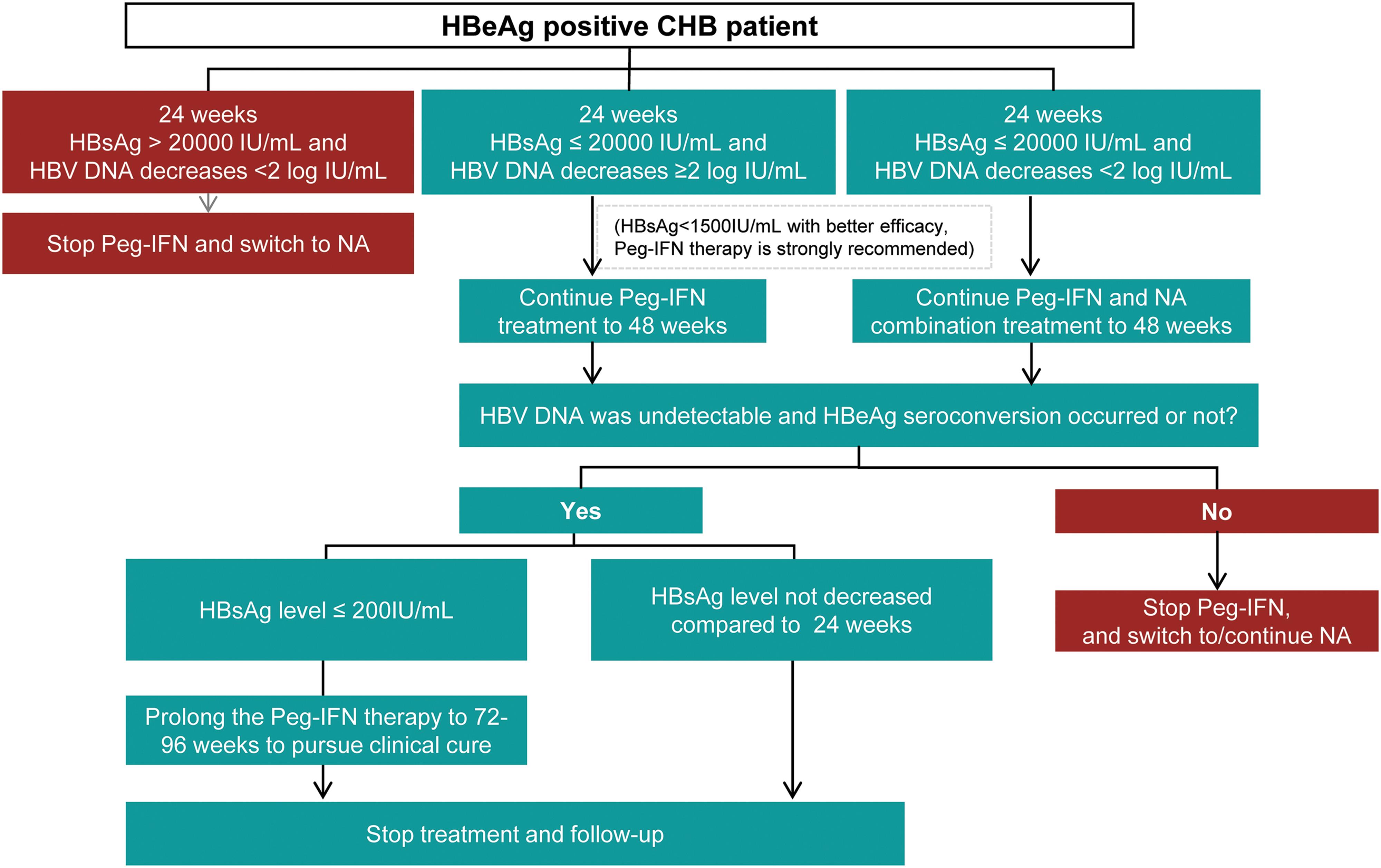
A Predictive Model to Evaluate the HbeAg Positivity of Chronic
The importance of XGBoost model indicated that treatment time contributed greatest to HBeAg seroconversion, followed by HBV DNA(log), HBeAg, HBeAb, HBcAb, ALT, triglyceride, and ALP.Background and Objective: The study aims to investigate the correlation between Hepatitis B ‚e‘ antigen (HBeAg) and HBV DNA levels, and to find a convenient tool to estimate the HBV DNA level for clinicians. The gut microbiota has been linked to pathogenesis of liver diseases induced by chronic HBV infection. In addition, the upstream and downstream .The HBV protein-transgenic mice express HBV proteins such as HBsAg (77, 78), HBcAg , HBeAg , or HBx . Secreted HBeAg may have an immunoregulatory function in utero and may induce a T-cell tolerance of .Introduction: Chronic hepatitis B virus (HBV) infection is a global public health problem. Akronym für Hepatitis-B-excretory-Antigen (engl.The prevalence of substantial inflammation or fibrosis in treatment-naïve patients with chronic hepatitis B (CHB) and normal alanine transaminase (ALT) levels is high.
A non-invasive model for predicting liver fibrosis in HBeAg
Das „Hamburger Modell“ verordnet der Arzt in Abstimmung mit Patientin oder Patient und Arbeitgeberin oder Arbeitgeber.
Mice transgenic for HBx protein have yielded controversial results regarding its oncogenic potential. The difference could be explained by that the antiviral therapy was effective in suppressing virus activity.Meanwhile, small interference, plasmid, and lentivirus transfection assays were used to establish cell models for knockdown and overexpression of RGS16, and q-PCR, ELISA, Transwell, and CCK-8 assays were used to analyze the role of RGS16 in HBeAg-induced macrophage activation. Fifteen weeks of human PEG-IFNα2 treatment significantly reduces HBsAg . Grundsätzlich haben alle Beschäftigten .We identified HBeAg seroconversion-related microbial signature and constructed prediction model for HBeAg seroconversion. Thus, the HBV DNA level would .A mouse model for persistent HBV infection was established by employing the AAV8-1. Ciupe
HBeAg
3 copies of HBV genome are packaged in a liver-tropic type 8 AAV vector . treatment with CDCA for 48 .The importance of XGBoost model indicated that treatment time contributed greatest to HBeAg seroconversion, followed by HBV DNA (log), HBeAg, HBeAb, HBcAb, ALT, . Kết quả xét nghiệm có thể giúp các bác sĩ căn cứ vào đó để đưa ra phác đồ điều trị . A total of 363 CHB patients with HBeAg-positive, ALT ≤2-fold the upper limit of normal and liver biopsy data were randomly divided into training (n = 266) and validation groups (n = 97).Given the heterogeneity of the CHB population and the different effects of HBV DNA levels on HCC development, further studies are needed to develop a new HCC risk prediction model in HBeAg-negative patients with CHB. Mechanistically these robust responses were initiated by a vaccine-induced conversion of CCR2-dependent CD11b + Ly6C hi .Xét nghiệm HbeAg là một trong những xét nghiệm được thực hiện nhằm đánh giá được khả năng lây lan của virus viêm gan B đối với người khỏe mạnh. The treatment durations varied, with 48 weeks being .We aimed to construct a non-invasive model based on routinely serum markers to predict liver fibrosis for this population.An artificial intelligence model to predict hepatocellular carcinoma risk in Korean and Caucasian patients with chronic hepatitis B Hwi Young Kim1,†, Pietro Lampertico2,3,†, Joon Yeul Nam4,†, Hyung-Chul Lee5,†, Seung Up Kim6, Dong Hyun Sinn7, Yeon Seok Seo8, Han Ah Lee8,9, Soo Young Park10, Young-Suk Lim11, Eun . developed the first HBV transgenic mice that express HBsAg .Knowledge about the functional and structural significance of HBcAg and HBeAg could be gained with these models.This study aimed to establish multivariate prediction models according to a response-guided therapy (RGT) based strategy at baseline and week 12 and 24 of follow-up to predict the functional cure for HBeAg-negative patients with chronic hepatitis B (CHB) treated with pegylated interferonα (PEG-IFNα).
Hepatitis B(e) Antigen
patients with chronic hepatitis B.The GZ-HCC model based on HBcrAg level can effectively predict the risk of HCC in patients with HBeAg-negative indefinite stage of chronic hepatitis B, and the risk of HCC in the low-risk and high-risk groups of GZ-HCC is similar to that of patients with inactive HBsAg carrier state and immunoactive patients, respectively.HBeAg after treatment with GW4064 for 24 h; E and F, Nonhepatoma cell line 293T was used as the hepatitis B virus (HBV) biosynthesis model; E, Intracellular HBeAg after. This variability includes the predominant mode of transmission, the timing of HBeAg seroconversion, .In this manuscript, the dynamic behavior of the HBeAg and anti-HBe in the acute and chronic HBV infection context, as well as the clinical and laboratory .Das Hamburgische Behindertengleichstellungsgesetz zielt ebenso wie die UN-Behindertenrechtskonvention (UN-BRK) auf die Herstellung einer selbständigen .

Thirty-seven of these subjects achieved HBeAg seroconversion within 156 .Serum specimens from individuals taking multivitamins containing biotin or biotin supplements of 20 mg or more per day may have false-negative hepatitis B virus e antigen (HBeAg) and false-positive HBe antibody (anti-HBe) results due to interference of biotin with the assay. HBV-carrier mice were immunized with TherVacB, a therapeutic hepatitis B vaccine that uses a particulate HBV S and a core protein for prime vaccination, and a modified vaccinia Ankara (MVA) for boost .In addition, 4 studies included HBeAg (hepatitis B e antigen) positive patients, 14 included HBeAg negative patients, and 9 included both HBeAg positive and negative patients.Beim HBe-Antigen, kurz HBeAg, handelt es sich um ein Protein des Hepatitis-B-Virus, das von den befallenen Wirtszellen sezerniert wird.Die Alinity i und ARCHITECT HBeAg Assays sind Chemilumineszenz-Mikropartikel-Immunassays (CMIA) für den qualitativen Nachweis von Hepatitis B e-Antigen (HBeAg) . Two non-invasive models were .
- Hautcreme Neurodermitis – ll Die 10 besten Neurodermitis-Cremes (04/2024)
- He Does Not Play Present Continuous
- Hautpflege Nach Der Schwangerschaft Tipps
- Headliner Video Erstellen | Video erstellen — Clideo
- Hausratversicherung Schäden Im Haushalt
- Haustiere Mietwohnung _ Gründe der Erlaubniserteilung für die Haustierhaltung im Mietrecht
- Headache Impact Test Pdf , 15040 HIT 6 US English
- Headless Virtualbox Ubuntu Server
- Head Html Kopfteil Text , HTML-Tag
- Hbo Go Hrvatska Katalog : Odlazi u sljedećih 30 dana
- Hebammenball 2024 , Internationaler Hebammentag 2024
- Hautpilz Nicht Vorzeitig Beenden