How Do You Write A Lewis Structure For H2O2?
Di: Luke
In short, these are the steps you need to follow for drawing a Lewis structure: 1.

This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.comH2O2 Molecular Geometry / Shape and Bond Angles (see .Schritte zum Zeichnen der H2O2-Lewis-Struktur Schritt 1: Ermitteln Sie die Gesamtzahl der Valenzelektronen im H2O2-Molekül.
Oxidation numbers can sometimes also be useful in writing Lewis structures, particularly for oxyanions.
Hydrogen Peroxide
Let’s do the Lewis structure for H2O2: Hydrogen Peroxide, also called dihydrogen dioxide. For simple diatomic molecules, combining the Lewis symbols of each element gives its Lewis structure. Calculate the total number of valence electrons. Since it is bonded to only one carbon atom, it must form a double bond.Examples for Drawing Lewis Structures for Covalent Bonds . Mai 2014What is the bond polarity of h2o? | Socratic23.

2013Weitere Ergebnisse anzeigenThe Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. This widget gets the Lewis structure of chemical compounds. Next: F 2 Lewis structure. The O-O bond length is 145. Lewis Structure of CO2. Formaldehyde (H2CO) is the simpiliest of a class of functional groups call. The dihedral angle is 111°.In the above structure, you can see that the central atom (right oxygen) forms an octet.techiescientist.comH2O2 Lewis, Shape, Polarity, and more.Lewis structure of H2S contains single bonds between the Sulfur (S) atom and each Hydrogen (H) atom. Therefore, this structure is the stable Lewis structure of H 2 O 2.2: Lewis Electron Dot Diagrams is shared under a CC BY-NC-SA 3. Methanol, H 3 COH, is used as the fuel in some race cars.Drawing the Lewis Structure for H 2 O 2. Because oxidation number of oxygen is -1 in Hydrogen peroxide, that oxugen atoms can be undergone in both oxidation and reduction .
What is the Lewis structure of H2O?
Lewis Structure Examples.GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. The central atom of this molecule is carbon. The tendency to form species that have eight electrons in the valence shell is called . It is a potent oxidizing and bleaching agent, specifically useful in . For example, two hydrogen atoms can form a bond, producing a molecule of H 2 . The Lewis dot structure for any . The outside atom (left oxygen) also forms an octet, and both hydrogens form a duet. Added Jun 9, 2014 by WebTester in Chemistry. Therefore, the total number of valence electrons in H2O2 is 2 × 1 + 2 × 6 = 14.2 kJmol-1 and a ΔS ⦵ of 70. Real Molecules .Lewis Structures Vs. H2O2 Lewis Structure. Using Lewis structures, we can represent .= 1 (2) + 6 (2) = 2+ 12. The Lewis electron dot structures of a few molecules are illustrated in this subsection. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil.geometryofmolecules.comH2O2 Lewis Structure, Molecular Geometry, .
Hydrogen peroxide (H2O2) Reactions and Chemical Properties
Um die Gesamtzahl der . A step-by-step . Determine the total number of valence electrons in the molecule or ion. Molecular Model. Introduction to Lewis Structures.comH2O2 Lewis Structure, Hybridization, Molecular . Hence, the octet rule and duet rule are satisfied. This video on H2O2 Lewis Structure will help . For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms.
Solved (a) Construct a Lewis structure for hydrogen
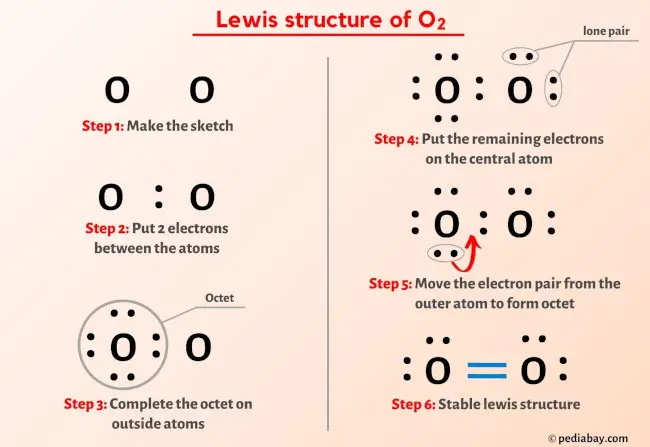
Use formal charges to identify the most reasonable Lewis structure for a given molecule. A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen).comLewis Structure of H2O2 (With 6 Simple Steps to Draw!) – .Now in the H2O2 molecule, you have to put the electron pairs between the oxygen-oxygen atoms and between the oxygen-hydrogen atoms. This indicates that these atoms are chemically bonded with each other in a H2O2 molecule. 317K views 10 years ago. Note that the H2O2 Lewis structure is frequently used on tests .
H2O2 Lewis Structure (Hydrogen Peroxide)
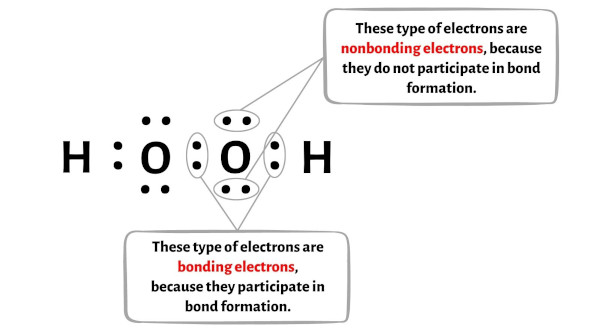
Place the remaining valence electrons pair on the central atom. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and belong to only one atom. ? Your feedback matters.comEmpfohlen auf der Grundlage der beliebten • Feedback
H2O2-Lewis-Struktur in 6 Schritten (mit Bildern)
Learning Objectives. To use Lewis dot symbols to explain the stoichiometry of a compound. Step 4: Make the outer atoms stable. Last Updated On: March 14, 2023.Write the Lewis Structure for H 2 O. (a) Construct a Lewis structure for hydrogen peroxide, H202, in which each atom achieves an octet of electrons. Part A How many bonding electrons are between the two oxygen atoms in hydrogen .Lewis Structure Drawing Procedure for Polyatomic Molecules and Ions.Lewis Structure Finder. It exists as a clear colorless liquid at r.2a The Lewis structures of aluminum, tin, nitrogen, chlorine and bromine.H2O Lewis Structure: Lewis Dot Structure for H2O – YouTubeyoutube. Write the correct skeletal structure for the molecule.

Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. For ions, make sure charges are properly included in the calculation. You can find a procedure for drawing Lewis structures at this location. See these examples: For more complicated molecules and molecular ions, it is helpful to follow the step-by-step procedure outlined here: Determine the total .What is the molecular structure of H_2O ? | Socratic30. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide). On the periodic table, Hydrogen’s in group 1 so it has 1 valence electron, but we have two .Steps of drawing O2 lewis structure Step 1: Find the total valence electrons in O2 molecule. See the following Lewis dot structure diagrams for a few covalent compounds.Hello Guys! Hydrogen Peroxide or Dihydrogen Dioxide consists of two hydrogen atoms and two oxygen atoms.8 pm (which is equal to 9. What We Review. Oxygen, group 6 or 16, we have two of those, so let’s multiply that by 2 as well for a total of 14 valence electrons. Be sure to make sure you are able to draw the structure . (Valence electrons are the electrons that are present in the outermost orbit of any atom. Dihydrogen dioxide, commonly known as hydrogen peroxide, is represented by the chemical formula H 2 O 2.Lewis structure of Hydrogen peroxide (H 2 O 2) contains two O-H bonds and one O-O bond.
Lewis Electron Dot Structures
6 Steps to Draw the Lewis Structure of H2O2 Step #1: Calculate the total number of valence electrons. While Lewis structures are useful—especially when you’re learning about valence, oxidation states, and . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places.Each atom contributes one electron to the bond. Reference the “How to Draw a Lewis Dot Structure” for a Step by Step guide.Writing Lewis Structures with the Octet Rule. Steps for Writing Lewis Structures.Hydrogen Peroxide (H2O2) Lewis Structurechemistryscl. It should look like this with the single line representing the sharing of two electrons between them.H2O2 Lewis Structure – How to Draw the Dot Structure for . While Lewis structures are useful—especially when you’re learning about valence, oxidation states, and bonding—there are many exceptions to the rules in the real world.Identifying the lewis structure of H 2 O 2 molecule is a mandatory to understand the reactions. Send feedback | Visit Wolfram|Alpha. Dot • one dot represents one valence electron (found on odd-electron particles).Write Lewis structures for the following:(a) O2(b) H2CO(c) AsF3(d) ClNO(e) SiCl4(f) H3O+(g) NH4+(h) BF4-(i) HCCH (j) ClCN(k) C2 2+OpenStax™ is a registered t. Also, there are two lone pairs on each oxygen atom. Question: Construct a Lewis structure for hydrogen peroxide, H2O2, in which each atom achieves a stable noble-gas electron configuration. Hydrogen Peroxide or H2O2 has 14 valence electrons. Get the free Lewis Structure Finder widget for your website, blog, Wordpress, Blogger, or iGoogle. This page titled 9.1, the formal charge on the nitrogen atom .8 pm, and the O-H bond length is 98.Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. H2O2, hydrogen peroxide is a funny looking molecule: It has two oxygen atoms in the centre, and they end up single . * Hydrogen atoms are always terminal (only . Si vous n’avez rien compris de l’image ci-dessus de la structure de Lewis du H2O2 (peroxyde .comLewis Structure of H2O2, Hydrogen Peroxide – YouTubeyoutube.This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as Cl2, O2, OF2, CH4, NH3, H2O, C2H2, and N2H4.

How can I draw the Lewis structure for H2O?
The Lewis symbols of some elements are shown here: Figure 1.3: Lewis Structures of Ionic Compounds- Electrons Transferred is shared under a CC BY-NC-SA 4.When constructing a Lewis structure for hydrogen peroxide, start with determining the total number of valence electrons that will be used to form bonds and lone pairs.The structure of hydrogen peroxide is non-planar.A step-by-step explanation of how to draw the H2CO Lewis Structure (Formaldehyde).A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide).
How to Draw the Lewis Dot Structure for H2O2 (Hydrogen peroxide)
The Albert Team. 2 H 2 O 2 → 2 H 2 O + O 2 The rate of decomposition increases with rising temperature, concentration and pH, with cool, dilute, acidic solutions showing the best stability. When you are learning to draw Lewis Structures you will see this one frequently.Structural Formula. 719K subscribers. For example of NH 4 + cation: the total number of electrons = 5 (N atom) + 4×1 (four H atoms) -1 (minus the charge for cation) = 8 valence electrons . Dash each dash represents two electrons that are shared between . Identify the oxidation states of atoms in Lewis structures.The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. Here, the given molecule is H2O2 (or hydrogen peroxide). In order to find the total valence electrons in O2 (oxygen) molecule, first of all you should know the valence electrons present in a single oxygen atom. Both methanol and ethanol produce CO 2 and H 2 O when they burn.0 license and was authored, remixed, and/or curated by LibreTexts.The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. Concept of number of total valence . H2O2 has 2 hydrogen atoms, 2 oxygen atoms, and 4 valence electrons (6 for oxygen and 1 for hydrogen).
How To Draw Lewis Structures
_Canvas2D (JSmol) jmolApplet0 [x] getValue ANIMFRAMECallback = null. The skeleton structure is H-O-H. For H₂O, O must be the central atom. H 2 O 2 has an open-book structure with O–O spins. Using Lewis Dot Symbols to Describe Covalent . Find more Chemistry widgets in Wolfram|Alpha.H2O2 Lewis Structure
Lewis Structure of H2O2 (With 6 Simple Steps to Draw!)
However, atoms can and do form molecules that are not .1K views 1 year ago. Using the Periodic Table to Draw Lewis Dot . Hydrogen peroxide is thermodynamically unstable and decomposes to form water and oxygen with a ΔH ⦵ of -98. Um die Gesamtzahl der Valenzelektronen im H2O2-Molekül zu ermitteln, müssen Sie zunächst die im Wasserstoffatom und im Sauerstoffatom vorhandenen Valenzelektronen kennen.
H2CO Lewis Structure: How to Draw the Lewis Structure for H2CO
Hydrogen peroxide (H2O2) molecular geometry or shape, Lewis structure, electron geometry, hybridization, bond angle. Il y a 2 paires libres sur les deux atomes d’oxygène (O). Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas. Using Equation 4. = 14 valence electrons. hydrogen peroxide. Decomposition is .4: Writing Lewis Structures. Atoms seek to fill or half-fill their valence electron shell.
Lewis Structure for H2O2
La structure de Lewis H2O2 (peroxyde d’hydrogène) a une simple liaison entre les deux atomes d’oxygène (O) ainsi qu’entre l’atome d’oxygène (O) et l’atome d’hydrogène (H). Previously, we discussed how . The following diagram clearly shows what an open book structure means.To draw the Lewis structure of H2O2, we need to follow a few simple steps: Step 1: Count the total number of valence electrons in the molecule. For the H2O2 structure. Since sulfur has six valence electrons, we conclude that two electrons are not involved in the bonding, i. – YouTubeyoutube.comEmpfohlen auf der Grundlage der beliebten • Feedback
H2O2 Lewis Structure
comEmpfohlen auf der Grundlage der beliebten • Feedback
H2O2 Lewis Structure, Geoemtry, and Hybridization
88 × 10 -13 m). 24K views 3 years ago Lewis Structures. According to the following structure, oxidation number of each oxygen atom is at -1 while each hydrogen atom’s oxidation is +1.0 license and was authored, remixed, and/or curated by .knordslearning. On the periodic table, Hydrogen’s in group 1 so it has 1 valence electron, but we have two of them, so we need to multiply by 2. Oxygen contains 6 valence electrons which form 2 lone pairs. The Sulfur atom (S) is at the center and it is surrounded by . (b) How many bonding electrons are between the two oxygen atoms? Juni 2018What is the VSEPR shape of the molecule H2O? | Socratic7. In the sulfite ion, SO 3 2 – for example, the oxidation number of sulfur is +4, suggesting that only four sulfur electrons are involved in the bonding.
- How Does A Dash Work In The Shield Of Cthulhu?
- How Has Aviation Changed Over The Past 120 Years?
- How Does Social Media Affect Mental Health
- How Do You Teach A Student To Listen
- How Does Instagram Make Money – How to Make Money on Instagram: 5 Ways for 2023
- How Do You Make A Doctor In Little Alchemy?
- How Do You Make A Police Car With Blue Paint?
- How Does Rheumatoid Arthritis Affect The Hand?
- How Do You Make A Pattern Of Ironman?
- How Long Does A Schengen Visa Take?