How Soap Works , Saponification Definition and Reaction
Di: Luke
Process of Making Felted Soap. Handwashing Science: Why Soap works? April 8, 2020 – By Ameline Lim, Ph.How soap works. Soap molecules’ tails surround the particles, creating a kind of mini jail cell that washes away when you rinse with water down the drain! If you want to learn the basics of SOAP, this tutorial is for you.Soap, which has been in around for thousands of years, 2 is uniquely structured to help destroy the virus. Web-transmitted data is usually structured in some way. The long hydrocarbon chain is of course non-polar and hydrophobic (repelled by water).How Soap Works. These elements come together like a symphony, with each playing a distinct role.This NBC News Learn video explains how soaps and detergents work to break up grease and dirt on soiled surfaces, by breaking water’s surface tension and susp. BrainPOP posted an episode of BrainPOP News.How does soap work??????:⏲ 0:00 Chemistry of soap molecules⏲ 0:33 How does soap work?⏲ 1:12 How does soap kill germs?⏲ 1:41 Bar soaps vs Liquid soaps??.Soap molecules have two ends: hydrophilic, attracting water, and hydrophobic, repelling water. List the benefits and problems with using soap.gl/fWlLjuSubscribe: http://goo.
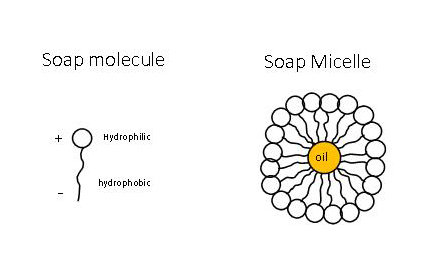
Soap Micelles Formation
An emulsifier is capable of dispersing one liquid into .
Why do we use soap?
This dual nature allows soap to perform its magical cleaning act by effectively breaking down and removing dirt, oils, and grime from surfaces.Soap acts as a surfactant—that means it works by lowering the surface tension between oils and grime and water, and emulsifying oils to allow them to be .
LAUNDRY SCIENCE 101: HOW SOAP AND DETERGENT WORKS
The soap molecules stand up on the surface as the polar carboxyl salt end is attracted to the polar water. Wash your hands! You’ve heard it a million times by now. Breaking news: germs are not invincible. Soap also has another function. Each hard water mineral molecule has 2 ions – so each hard water molecule can take 2 soap molecules (which has 1 ion). One end of it is polar and the other is non-polar, allowing it to emulsify grease into water. Learning Objectives., sodium hydroxide or potassium hydroxide) in water and also heat. A drop or two of soap in water forms a monolayer (Figure 14. Washing with soap and water is an effective way to destroy and dislodge many microbes, including the new coronavirus.Washing your hands is very important but why do we have to wash our hand with soup? Ben shows Evie a very clever experiment that show you how soap works!CBe. Wrap the wool not only lengthwise but widthwise also and even at the corners too.Often we use the words soap and detergent interchangeably, but really they’re quite different things.Video ansehen5:48This NBC News Learn video explains how soaps and detergents work to break up grease and dirt on soiled surfaces, by breaking water’s surface tension and susp. This chemical reaction is known as saponification. It is necessary to cover the soap evenly. XML (or Extensible Markup Language) is a text format that establishes a set of rules to structure messages as both human- and machine-readable records. Fats and oils are the main characters in our soap opera. Swishing the soapy water around allows the soap or detergent to pull the grime away from clothes or dishes and into the larger pool of rinse water.09M subscribers. Soap can bond to the oil molecules and then pull them away from a surface as it is carried off by water. XML (or Extensible .
Handwashing 101: How Does Soap Work? — Konsyse
With soap bar production, the formula is then pressed into .

In this video, Twinkl Teacher Ashley demonstrates how a simple experiment can be set up in school or at home to visibly show the effect of soap. Neither detergents nor soaps accomplish anything except binding to the soil until some mechanical energy or agitation is added into the equation. Let the soap sit on your skin for 2-3 minutes before rinsing it off with warm water.How Soap Cleans
How does soap work?
How do detergents and soaps work?
, causing scaling or fissures to develop in the . With the latest episode of #BrainPOPNews, you can teach your kids exactly how washing hands with soap helps keep everyone safe.The hard water ions deactivate the surfactant by binding to it instead of the dirt. The salt end of the soap molecule is ionic and hydrophilic (water soluble). Avoid prolonged sun exposure while using Kojic acid soap, as it can make your skin .The non-polar hydrocarbon end of the soap molecule is repelled by water.Soap works because of the power of intermolecular forces. A detergent is a chemical substance you use to break up . What is a Soap Web Service2. why? What exactly is soap, and how does it get all the dirt o. Making Soap: • Making Soap In this video, we explore the .
Handwashing Science: Why Soap works?
How does Soap Work? NileBlue. 5) on the water surface as shown in the graphics on the left. 58 · 3 comments · 4.

Soaps are mixtures of sodium or potassium salts of fatty acids which can be derived from oils or fats by reacting them with an alkali (such as sodium or potassium hydroxide) at .
How Soap Works: The Science Behind Handwashing
Web Services Beginner Tutorial 4 – What are SOAP Web ServicesToday we will learn:1.MICELLESSoaps are molecules having two ends with differing properties, one end is hydrophilic, that is, it dissolves in water, while the other end is hydroph.gl/oDnOPVShare on Twitter: http://goo. They consist of molecules called triglycerides, which have three fatty acid chains.How Detergents Work . The soap molecules will pry oil molecules off of surfaces and suspend them within water, which can now wash them away. REST (Representational . The reaction is used commercially to make soap, lubricants, and .Any time your clothes or body get dirty, you can wash them and make them nice and clean! But.Soap is a simple but powerful tool to fight germs and viruses. · April 30, 2020 · Follow. When the ions attract, the soap becomes insoluable and can’t bind to the dirt. Apidog also supports other protocols, such as REST, and integrates with popular tools like . This is why you may need up to four times the recommended .Work up a lather with the soap and apply it to your skin.
Saponification Definition and Reaction
The most common and best known function of soap is to help in removal of dirt which cannot be removed by water alone. • Handwashing with soap essentially pulls off grease and dirt from the skin or any particular surface, while allowing them to become suspended in water.Soap is a blend of simple yet effective ingredients: fats or oils, alkalis, and water. Soap Web Services Specifications/Components_. First, place the soap bar in the roving wool and wrap it around the soap tightly.Autor: NBC News LearnSaponification is the name of the chemical reaction that produces soap. Germs stick to the oils and grease on our hands (sounds yucky, but it’s totally normal).Soap works primarily by removing, not by killing, though some germs are killed in this process. You will learn what SOAP is, how it works, and how to use it with Apidog, a platform that helps you create, test, and document APIs. Use Kojic acid soap once a day, preferably at night.SOAP (also known as Simple Object Access Protocol) is a secure way to build APIs, and it works by encoding data in the XML format. Learn how it works and why it is so effective in this article from BBC Science Focus Magazine.When grease or oil (non-polar hydrocarbons) are mixed with a soap- water solution, the soap molecules work as a bridge between polar water molecules and non-polar oil molecules. For more about . Moisturize your skin after using Kojic acid soap to prevent dryness.We all use soap. How does Soap Work. “Soap works because it’s the right structure to do the job,” says . You might wonder why it’s essential to delve into the science of how soap works .Soap’s water-hating, oil-loving tails poke their way into this membrane and break it apart. Though soap became commonly used around 1000 years ago, . Soap traps the virus (and other dirty, germy particles) in a little bubble called a micelle.The chemical structure of soap works to unstick dirt and germs from our skin and can even destroy viruses completely. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Since soap molecules have both properties of non-polar and polar molecules the soap can act as an emulsifier. Find out the difference between soap and detergent, and the types of soap available for various .

soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as the human skin, textiles, and other solids.How does soap make things clean? Here’s what science has to say: Before we can talk about how soap makes things clean, first consider: What does it mean to be .Soap is produced by combining and then heating oil and lye.gl/ZYI7GtVisit our s.SOAP works with XML only. Soap needs some time and action to get itself situated in all those tidy micelle . The reaction requires a solution of an alkali (e.Hence, because they are also emulsifiers, soaps suspend dirt-containing oil, grease, and grime in such a way that they can be removed and washed away with water.
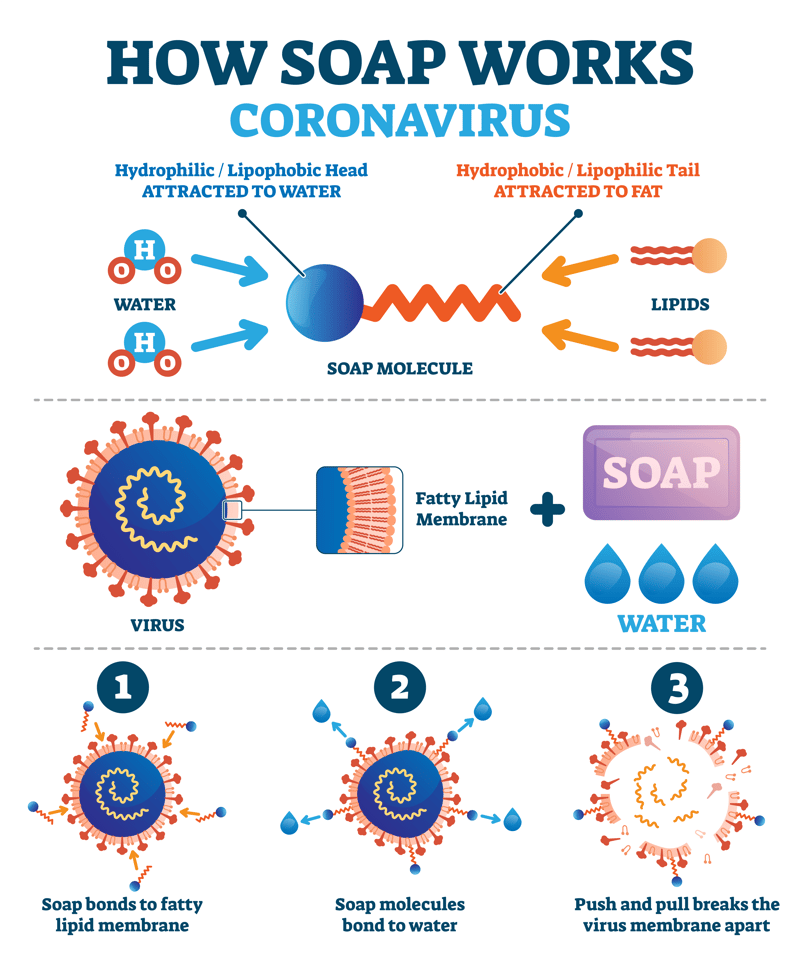
Soap molecules dislodge dirt, oil, and grease particles – and the disease-causing germs they carry – from your hands, one micelle at a time. The cleansing action of soap is determined by its polar and non-polar structures in conjunction with an application of solubility principles.
303K views 6 years ago. These dual-properties allow the molecules in soap to bond with oil and water. Examples of soap and . But how does it work?Share on Facebook: http://goo. Rinsing washes the .1: Cleaning with Soap.Table of Contents Liquid, solid or powder, all soaps work similarly Soap is so easily available nowadays that we often take it for granted. When soap and water are not available for hand washing or when repeated hand washing compromises the natural skin barrier (e. The thickness of the roving must be ¼ inch for perfect covering.
Understanding How Detergents Actually Work
[ Source] When you wash your hands with soap, it dislodges the dirt, grease, oils, and disease-ridden fecal matter particles on your hands by creating these micelles.
How soap works
Describe the mechanism by which soaps exert their cleansing action.SOAP is a widely used protocol for exchanging messages over the web.Soap molecules possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) ends.

The alkaline substance that helped create it gives it a polar “head” at one end.Learn how soap is made, how it works, and how it evolved over time. Grease, oils and other organic .How Soap Works | BrainPOP News.
Why Soap Works
Soap doesn’t kill germs on our hands, it removes them.A soap molecule is perfectly suited to mixing oil and water because it shares some qualities of each.
How Soap Works
First, the hydrophilic ends of the soap molecule attach to the water, . The two most popular data formats are XML and JSON.
- How Old Was Mac Daddy When He Died?
- How Much Does It Cost To Get A Steam Level?
- How To Change Whatsapp Ringtone On Android
- How To Clean Inside Car Windows Without Streaks
- How To Brush Your Tooth | How to Brush: A Guide to Perfect Technique
- How Tall Is Blackpink’S Lisa? : Lisa height
- How Much Does A Lottery Cost In The Uk?
- How Old Is Leia In A New Hope?
- How To Adjust Sensitivity On Razer Mamba Wireless?
- How To Connect Alexa Speakers , How to Pair Alexa With a Bluetooth Speaker
- How To Choose A Neck Tattoo Design?
- How Old Was John Keays-Byrne When He Died?