Hydrogen Alkali Group 1 _ Why is Hydrogen in Group 1? (+ 3 Facts to Know)
Di: Luke
For example, sodium reacts with water: Sodium + water → sodium hydroxide + hydrogen. Although often listed in Group 1 due to its electronic configuration, hydrogen is not technically an alkali metal since it rarely exhibits . When they react with water, Group 1 elements form metal . Group 1: Properties of Alkali Metals. The akali group formally contains hydrogen (H) even so this element is not counted among the alkali metals and thus not listed below. In each case, there is one electron in the outer orbital and that is an s-orbital electron. so a proper position is not assigned to it in periodic table.Descriptive Chemistry.
Group 1: Hydrogen And Alkali Metals
; Hydrogen can be placed in group 17 (ns 2 np 5) (halogens) as it can get one electron to get outer electronic configuration of inert gas.53 sodium Na 11 23 1 98 solid 157 0.
Group 1 Elements
Also note the green H above the alkali metals.

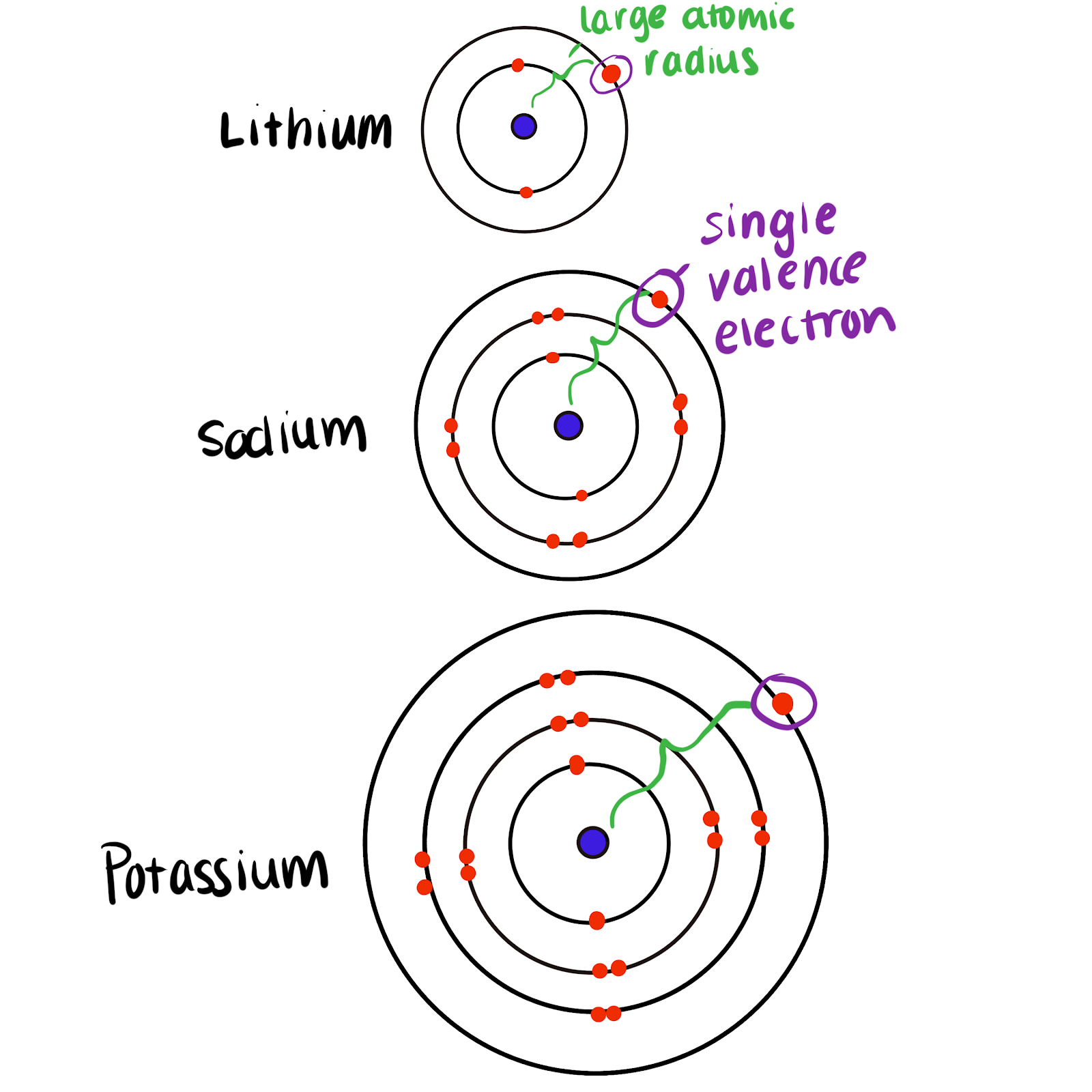
Electronic Configuration: The electronic configuration of hydrogen is $$1s^1$$ which is similar to the outmost electronic configuration of alkali metals $$(ns^1)$$. All the Group 1 elements are very. Hydrogen accounts for much of the universe – about 93 %.Hydrogen is a chemical element; it has symbol H and atomic number 1.Group 1 metals exhibit high chemical reactivity. The alkali metals are so called because reaction with water forms alkalies (i.If we look at Group I (red column), we see that it is labeled alkali metals. It includes the nonmetal hydrogen (H) and six metals that are called alkali metals.Alltogether the alkali metals provide .There are following similarities of hydrogen with alkali metals 1. hydrogen and alkali metals of group 1 react with copper (ii) oxide to give copper give reason.
s-Block Elements
Reactivites of metals towards O 2 and H 2 O increases down the column.We can, however, predict what its properties might be by exploring the trends in the group.Hydrogen and Alkali Metals. They form alkaline solutions when they react with water. This is why they are called alkali metals.Group 1 is the first group in the periodic table containing elements that are commonly known as the Alkali metals.
Answer: Electronic configuration of hydrogen is 1s 1 which is similar to the outer electronic configuration of alkali metals of group 1 i. (with a pH above 7).
3 Selected Properties of the Group 1 Elements.97 potassium K 19 39 1 63 solid 203 0.Group 1 Elements.periodictableguide.Group 1 / IA; Alkali Group. Nitric acid in the dilute form is not used in the laboratory preparation of hydrogen from metals.86 rubidium Rb 37 85 1 39 solid 216 1. Add to Library. The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table. Please update your bookmarks accordingly.ukEmpfohlen auf der Grundlage der beliebten • Feedback
Group 1 / IA; Alkali Group
Hydrogen is not an alkali metal itself, but has some similar .Group I actually contains also the alkali metals but hydrogen (H) is not counted among them and thus rates a separate page., strong bases capable of neutralizing acids). Group 1: Hydrogen and the Alkali Metals. Lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium . All Group 2 metals all react similarly, burning to form oxides (compounds containing the O 2-ion) as shown: \[2 M(s) + O_2(g) \rightarrow 2 MO(s) \label{10} \] Once initiated, the reactions with oxygen are .
These s-block elements are found in Group 1 and Group 2 of the periodic table and are the most active metals. These are very reactive metals and have to be .
Why is hydrogen not grouped in a family?
; It is a diatomic gas.By knowing which group an element is in, we can determine the number of reactive electrons and say something about how that element will behave. 1 The Isotopes of Hydrogen compares the three isotopes of hydrogen, all of which contain one proton and one electron per atom. This page discusses the trends in .1: The Alkali Metal Elements; 3.Why group 1 elements are also called alkali metals – . Potassium (K) . The decomposition temperatures again . Give reason for the following.Group 1 elements and their properties. Hydrogen, the most abundant element in the universe, is the ultimate source of all other elements by the process of nuclear fusion. All metals are malleable and they become softer down to column. Alkali metal salts are prepared . In fact, in many ways it is more similar to its diagonal neighbor magnesium (Mg) than .Alkali Metals – Properties, Electronic Configuration, Periodic .
Group 1

Group 1: Hydrogen And Alkali Metals | Periodic Table Guide.Group 1/ I; Hydrogen Group I actually contains also the alkali metals but hydrogen (H) is not counted among them and thus rates a separate page. Elements Organized by Group. All Modalities. Group 3-12: Transition and Inner transition metals group. To better organize out content, we have unpublished this concept. As a result of their low first ionization energies, the alkali metals . Click Create Assignment to assign this modality to your LMS. Out of these elements except for hydrogen, the remaining elements are popularly known as the Alkali metals.comEmpfohlen auf der Grundlage der beliebten • Feedback
Group 1/ I; Hydrogen
Group 15: Nitrogen group. Electropopsitive Character: Hydrogen can from unipositive ion by losing . Group 1: Alkali metals group (hydrogen not included) Group 2: Alkaline earth metals group.Hydrogen is placed in Group 1 of the periodic table because it has a single electron in its outer shell, similar to the other alkali metals. Als Alkalien (über lat.Group 1 – The Alkali Metals | The Periodic Table – YouTubeyoutube. Physical properties of the Alkali Metals. Group 14: Carbon group.All alkali metals react with hydrogen at high temperatures to produce the corresponding hydrides, and all reduce water to produce hydrogen gas.Group 1 elements also called alkali metals.The group 1 metals are known as the alkali metals.comWhy are group 1 elements called alkali metals? – Periodic . Hydrogen accounts for much of the universe – . Som it is placed with alkali metals in group $$1$$ 2. It includes the nonmetal .Hydrogen easily donate the electron and forms a stable cation (H +) hence it resembles alkali metals of group. Summary of Alkali Metal (Group 1) Trends: 1. Helium (He) provides a bit less than 7 %; the rest thus can be seen as trace elements; the ash of burnt-out stars. The rest of the Group 1 carbonates do not decompose at laboratory temperatures, although at higher temperatures this becomes possible.; However, 1s 1 also resembles the outer electronic configuration of group 17 elements i.Alkali metals are a class of chemical elements found in Periodic Table Group 1.
Elements of Group 1 and 2
Why is hydrogen not group 1 or 7? Hydrogen can share electrons to form covalent bonds and gain electrons to form hydrides; which differentiates it from the Group 1 elements. However, it varies greatly from the alkali metals as it . They must be stored under oil to keep air and water away from them.: Jupiter and Saturn, the big planets of . 1 It also has similar chemical . The most common isotope is protium ( 1 H or . In this article, we discuss Group .
1 Alkali metals are highly reactive metals, while hydrogen is a gas. Periodic Table, S-Block / By Arun Dharavath.
Group 1: Hydrogen and the Alkali Metals
The alkali metals react with water to produce a metal hydroxide and hydrogen.
However, the ionic character of the group 1 metal hydrides decreases from LiH to CsH.Isotopes of Hydrogen.Sodium (Na) is an element in group 1 of the periodic table of the elements.
Alkali metal
Various properties of the group 1 elements are summarized in Table 21.
Why is Hydrogen in Group 1? (+ 3 Facts to Know)
Ionic hydrides form when hydrogen reacts with s-block metals, not including Be and Mg.There are total 18 different groups in Periodic table.Alkali metals are the chemical elements found in Group 1 of the periodic table. Group 13: Boron group.Truro School in Cornwall.In Group 1, lithium carbonate behaves in the same way, producing lithium oxide and carbon dioxide: Li2CO3(s) → Li2O(s) + CO2 L i 2 C O 3 ( s) → L i 2 O ( s) + C O 2.

3: Group 1
Know This : Hydrogen can be placed in group 1 as it has one electron in the outer shell, (1s 1). They are all metals and increase in reactivity . The alkali metals include: lithium, sodium, potassium, rubidium, cesium, and francium. Potassium (K) and sodium (Na) are ubiquitous, while rubidium (Rb), lithium (Li) and cesium (Cs) are rarer.Sodium and potassium are the .comperiodic table – Why are group 1 elements called alkali metals . Group 1 metals are referred to as alkali metals and have a charge of +1 Group 2 metals are referred to as alkaline earth metals and have a charge of +2. Elements in the same group of the periodic table have the same number of valence electrons. Francium (Fr) is radioactive and not found in nature.alkali metal, any of the six chemical elements that make up Group 1 (Ia) of the periodic table—namely, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).Group 1: Hydrogen and the Alkali Metals Alkali metals are the chemical elements found in Group 1 of the periodic table. In keeping with overall periodic trends, the atomic and ionic radii increase smoothly from Li to Cs, and the first ionization energies decrease as the atoms become larger. Among the eighteen groups of the Periodic table, Group 1 consists of Hydrogen (H), Lithium ( Li), Sodium (Na), Potassium (K), . Group 1 – The Alkali Metals.Similarly to Group 1 oxides, most group 2 oxides and hydroxides are only slightly soluble in water and form basic, or alkaline solutions. Granulated zinc is preferred to metallic zinc in the preparation of hydrogen using dilute acid.
Reactions of Main Group Elements with Hydrogen
Well, this was just a simple answer. Group-1 Elements of the Modern Periodic Table consist of Hydrogen (H), Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs), and Francium (Fr).53 caesium Cs 55 133 1 28 .
Hydrogen and alkali metals of group 1
Characteristics of Group 1 of the periodic table. Natriumcarbonat) werden Substanzen bezeichnet, die mit Wasser alkalische Lösungen . We have a new and improved read on this topic. All of these elements have a similar configuration of outer-shell electrons (see table below).orgGroup 1 Elements – Alkali Metals, Properties, Trends, Uses – . sal alkali von arab.1) 2) alkali metals 3) name symbol atomic number relative atomic mass number of electrons in outer shell melting point (°C) state at room temperature atomic radius (pm) density (g/cm3) lithium Li 3 7 1 181 solid 123 0. Need help getting ahead in Chemistry? Knowing your periodic table is the first step. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, [10] but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen.
The Alkali Metals (Group 1)
All the alkali metal hydrides are ionic solids with a high melting point.Hydrogen is not an alkali metal, but it is placed in the same group in the periodic table because it has one electron in its outer shell. The alkali metals share similar characteristic chemical properties because they each have one .; By adding one . But there are few more things to know about this topic which will make your concept super clear. It is colorless, odorless, tasteless, [11] non-toxic, and . Hydrogen accounts for much . H 2 O rxn: Li slow, Na vigorously, K evolves H 2, Rb/Cs explosive O 2 rxn (heat) products . Because hydrogen resembles proprties of both alkali metals (1st group)and halogen family (17th group) .This page discusses a few compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and cesium), including some information about the nitrates, carbonates, . The Hydride of lithium is more stable as compared .3: The Anomalous Chemistry of Lithium While lithium shows many properties that are clearly consistent with its position in Group 1, it also has key differences to the other alkali metals.When they react with water, Group 1 elements form metal hydroxides which are alkaline close alkaline Having a pH greater than 7. Although often listed in Group 1 due to its electronic configuration, hydrogen is not technically an alkali .
Group 1: Properties of Alkali Metals
Hydrogen shows similarity with alkali metals as well as halogens.Hydrogen is placed above group in the periodic table because it has ns 1 electron configuration like the alkali metals.2: Compounds of the Alkali Metals; 3. This is due to.comPhysical properties of the alkali metals – Group 1 – BBC . القلية / al-qalya / ‚ Pottasche ‘; Soda bzw. Various properties of the group 1 elements are summarized in Table 21. Li, cut w/ knife; Rb/Cs consistency of putty. Click here to view We have moved all content for this concept to for better organization. The reactivity of alkali metals with hydrogen decreases from Li to Cs.General Properties of the Alkali Metals. This group (column) of the table is shown in Figure below.
- Hypovereinsbank Eigenkapital Entwicklung
- Hyuna Pop Singer : HyunA-Profil, Alter, Geburtstag, Größe und (aktualisierte Fakten!)
- Hydrogen Stations Live _ Le réseau
- Hz Isa Hayatı Hakkında _ Hazreti İsa’nın Hayatı
- Hut Pork Pie , Pork-Pie-Hüte Herren
- Hysterie Anzeichen | Sigmund Freud: Zur Psychotherapie der Hysterie
- Hyundai 7 Sitzer _ Hyundai STARIA gebraucht kaufen bei AutoScout24
- Hyperurikämie Ernährung – Harnsäure senken: so geht`s!
- Hyperlink Warnmeldung Windows 10
- Hydra Dominatus _ Hydra Dominatus! : r/alphalegion
- Hydroxyzin Bluefish – Hydroxyzin Bluefish 25 mg Filmtabletten: Beipackzettel
- Hunger Betroffenen Menschen Weltweit