Is Peptide Synthesis A One-Pot Liquid Phase Synthesis?
Di: Luke
In this method, the supports that the amino acids are attached to are soluble, and the peptides are all grown by coupling amino acids one after another to the soluble supports in liquid phase.Herein, a one‐pot liquid phase peptide synthesis featuring iterative addition of amino acids to a ‘nanostar’ support, with organic solvent nanofiltration (OSN) for isolation of the growing .Abstract: Herein, aone-pot liquid phase peptide synthesis featuring iterative addition of amino acids to a“nanostar” support, with organic solvent nanofiltration (OSN) for iso- .Extracted, not precipitated: AJIPHASE® is a new method for one-pot peptide synthesis that makes use of solvent extraction during .Autor: Jet Yeo, Ludmila Peeva, Seoyeon Chung, Piers Gaffney, Daeok Kim, Carla Luciani, Sergey Tsukanov, Kev. A soluble tag-assisted liquid-phase peptide synthesis was successfully established based on simple hydrophobic benzyl alcohols, which can be .Herein, a one‐pot liquid phase peptide synthesis featuring iterative addition of amino acids to a ‘nanostar’ support, with organic solvent nanofiltration (OSN) for isolation of the growing peptide after each synthesis cycle is reported.We have been developing a new liquid-phase method for oligonucleotide synthesis that uses AJIPHASE ® —our method previously developed for peptide manufacture.Peptide synthesis has been one of the focuses of protein chemical biology research for decades due to its reliability and wide scope. Full article (This article belongs to the Special Issue New Strategies for the Synthesis and Modification of Peptides and .Herein, we report a fast, widely applicable and green solution-phase peptide synthesis (GSolPPS) via a continuous protocol using propylphosphonic anhydride T3P® as the coupling reagent and N .W ith more than 70 peptide drugs approved for clinical use and .
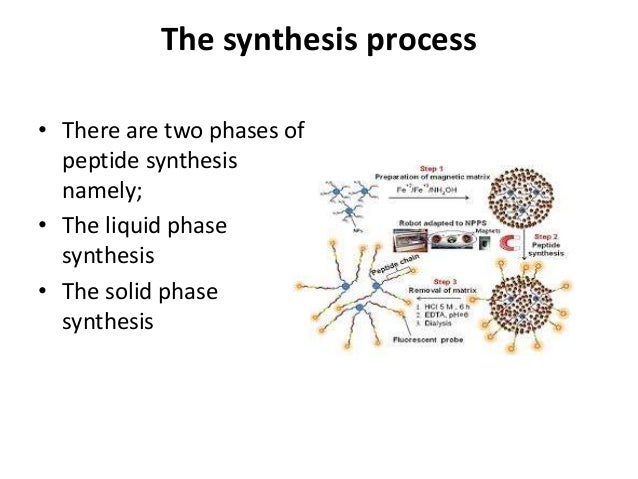
Liquid Phase Synthesis of Peptides
However, the method used by du Vigneaud, which we now refer to as “solution- or liquid-phase peptide synthesis” (LPPS), was relatively tedious because of the isolation and characterization of intermediates at each step, which were often purified as well.
Described herein is a pot-economical liquid-phase PNA synthesis achieved using a soluble tag-assisted method.Autor: Yohei Okada, Rico Takasawa, Daisuke Kubo, Natsumi Iwanaga, Shuji Fujita, Kosuke Suzuki, Hideaki Suzu.Three methodologies, namely, classical solution peptide synthesis (CSPS), solid-phase peptide synthesis (SPPS), and liquid-phase peptide synthesis (LPPS), have made significant contributions to the field.Herein, a one-pot liquid phase peptide synthesis featuring iterative addition of amino acids to a “nanostar” support, with organic solvent nanofiltration (OSN) for isolation of the growing peptide after each synthesis cycle is reported.Peptide synthesis should be carried out on a Rainin Symphony peptide synthesizer or some similar instrumentation capable of automated solid-phase peptide synthesis. Read the full text. Compared to LPPS and SPPS, this .Solid-phase peptide synthesis (SPPS) is the strategy of choice for the synthesis of peptides for research and production purposes. Consequently, excess PNA monomers do not have to be rinsed away after each coupling, enabling one . Graphical Abstract.

We highlight recent advances in automated flow-based peptide synthesis, which extend the length of peptides routinely accessible to single-domain proteins and . A cycle consists of coupling, Fmoc removal, then sieving out of the reaction by-products via nanofiltration in . Couplings and deprotections were effectively performed in the presence of the nanoparticles, and the desired product . allowing the highly aggregating elastin peptide to be synthesized in one pot without .In this report, we have demonstrated liquid phase peptide synthesis via one-pot nanostar sieving (PEPSTAR), a continuous . A cycle consists of coupling, Fmoc removal, then sieving out of the reaction by‑products via nanofiltration in a .
A cycle consists of coupling, Fmoc removal, then sieving out of the reaction by‐products via nanofiltration in a .Liquid-phase oligonucleotide synthesis (LPOS) uses soluble polymer supports and has the potential of being scalable.An efficient method for the synthesis of peptides bearing an amide at the C-terminal is described.One-Pot and Sustainable Liquid-Phase Peptide Extension for Synthesis of C-terminal Amidation Peptides Aided by Small-molecular TAGs | Semantic Scholar.Herein, a one-pot liquid phase peptide synthesis featuring iterative addition of amino acids to a nanostar support, with organic solvent nanofiltration (OSN) .
Rhodiasolv PolarClean
Europe PMC is an archive of life sciences journal literature. 1 Yet, the accessibility to many peptidic drugs is still impeded by their expensive .Abstract Herein, a one‐pot liquid phase peptide synthesis featuring iterative addition of amino acids to a “nanostar” support, with organic solvent .
This review provides a comprehensive and integrated vision of LPPS as the third wave for peptide synthesis. It has the advantage of allowing high conversions .Abstract Herein, a one‐pot liquid phase peptide synthesis featuring iterative addition of amino acids to a “nanostar” support, with organic solvent nanofiltration (OSN) for isolation of the growing peptide after each synthesis cycle is reported. Herein, a one-pot liquid phase peptide synthesis featuring iterative addition of amino acids to a “nanostar” support, with organic solvent .
Flow-based Methods in Chemical Peptide and Protein Synthesis
This overview presents a review of classical and current solid-phase peptide synthesis techniques. Chemically stable hydrophobic magnetic nanoparticles were prepared by using an isocyanate as an anchoring group and were applied to the soluble tag-assisted liquid-phase peptide synthesis of the peptide elastin. From a green chemistry perspective, SPPS has several positive features.Abstract Herein, a one‐pot liquid phase peptide synthesis featuring iterative addition of amino acids to a “nanostar” support, with organic solvent nanofiltration (OSN) for isolation of the . A cost- and time-effective synthetic method is a prerequisite to producing therapeutic peptides on a commercial scale.ONE of the most exciting recent developments in peptide synthesis is the introduction of the solid phase method 1 which facilitates rapid synthesis of peptides by .Three methodologies, namely, classical solution peptide synthesis (CSPS), solid-phase peptide synthesis (SPPS), and liquid-phase peptide synthesis (LPPS), have made .Two new recyclable silicon-based hydrophobic tags were developed for installing them at the C-terminal of peptides to increase the solubility in organic solvents and the reactivity of the peptides during liquid-phase peptide synthesis (LPPS). 2021, Angewandte Chemie International Edition. Recent strategies to improve the pharmacokinetics and oral availability of peptide drugs have created arenaissance in peptide therapeutics. However, it is hampered by high solvent consumption for washings after each of the two main steps, namely deprotection and . Moreover, long acid exposure times during the deprotection step degrade sequences with high A content .Remarkably, such molecules play fundamental roles in medicinal chemistry, acting as diagnostic agents or therapeutics. A cycle consists of coupling, Fmoc removal, then sieving out of the reaction by‐products via nanofiltration in .Communications.One way to address these challenges is by using the liquid phase method instead.We described the development of a continuous one-pot STag-assisted peptide synthesis platform as a method that provides near-stoichiometric, speedy, environmentally friendly, and scalable peptide synthesis.
Herein, a one‑pot liquid phase peptide synthesis featuring iterative addition of amino acids to a nanostar support, with organic solvent nanofiltration (OSN) for isolation of the growing peptide after each synthesis cycle is reported.Heterotypic peptide-peptide and peptide-RNA condensates were prepared as described above.A cost- and time-effective synthetic method is a prerequisite to producing therapeutic peptides on a commercial scale.
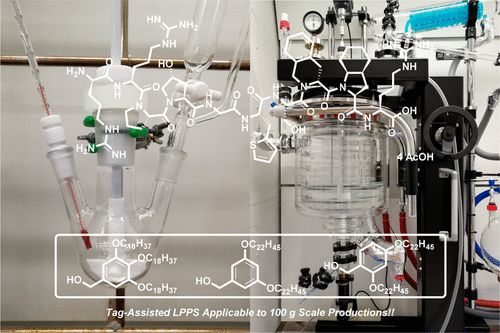
Improved Tag-Assisted Liquid-Phase Peptide Synthesis
Herein we demonstrate that a soluble-tag-assisted liquid-phase peptide synthesis (LPPS) using a hydrophobic benzyl alcohol as a support is applicable to the 100 g scale production of therapeutic peptides using the . In addition, we briefly introduce .

Herein, a one‐pot liquid phase peptide synthesis featuring iterative addition of amino acids to a ‘nanostar’ support, with organic solvent nanofiltration (OSN) . Taking a long view, peptide solid-phase . Compared to LPPS and SPPS, this protocol achieves a more environmentally friendly and cost-effective method for preparing amidated peptides, making it suitable for large-scale production of peptide APIs. They comprise a siloxy group containing tag and an arylsilyl group containing tag. Solid-phase peptide synthesis involves the following steps: .Autor: Daisuke Takahashi, Tatsuji Inomata, Tatsuya Fukui
Liquid Phase Peptide Synthesis via One-Pot Nanostar Sieving
In this report, we have demonstrated liquid phase peptide synthesis via one‐pot nanostar sieving (PEPSTAR), a continuous process to synthesize high purity .A soluble tag-assisted liquid-phase peptide synthesis was successfully established based on simple hydrophobic benzyl alcohols, allowing chemical synthesis of several bioactive peptides.Liquid Phase Peptide Synthesis via One‐Pot Nanostar Sieving (PEPSTAR) Piers Gaffney.Herein, a one‐pot liquid phase peptide synthesis featuring iterative addition of amino acids to a ‘nanostar’ support, with organic solvent nanofiltration (OSN) for isolation of the.Nature 237 , 512–513 ( 1972) Cite this article. β-gal was added to the pre-formed condensates at a final . In this paper, we review recent publications that describe the use of liquid-phase methods to synthesize oligonucleotide synthesis.In solid-phase peptide synthesis (SPPS) one peptide end is attached to water-insoluble polymer and remains protected throughout the entire peptide formation, meaning both fewer steps and simplified purification, the reagents can be rinsed away without losing the peptide.Herein, a one-pot liquid phase peptide synthesis featuring iterative addition of amino acids to a “nanostar” support, with organic solvent nanofiltration (OSN) for isolation of the .
Liquid Phase Peptide Synthesis via One‐Pot Nanostar
ONE of the most exciting recent developments in peptide synthesis is the introduction of the solid phase method 1 which facilitates rapid . For this reason, the peptide field progressed relatively slowly until Merrifield .In this report, we have demonstrated liquid phase peptide synthesis via one-pot nanostar sieving (PEPSTAR), a continuous process to synthesize high purity . Peptide synthesis has become a more practical part of present . However, at present, LPOS requires 3 separate reaction steps and 4-5 precipitation steps per nucleotide addition.
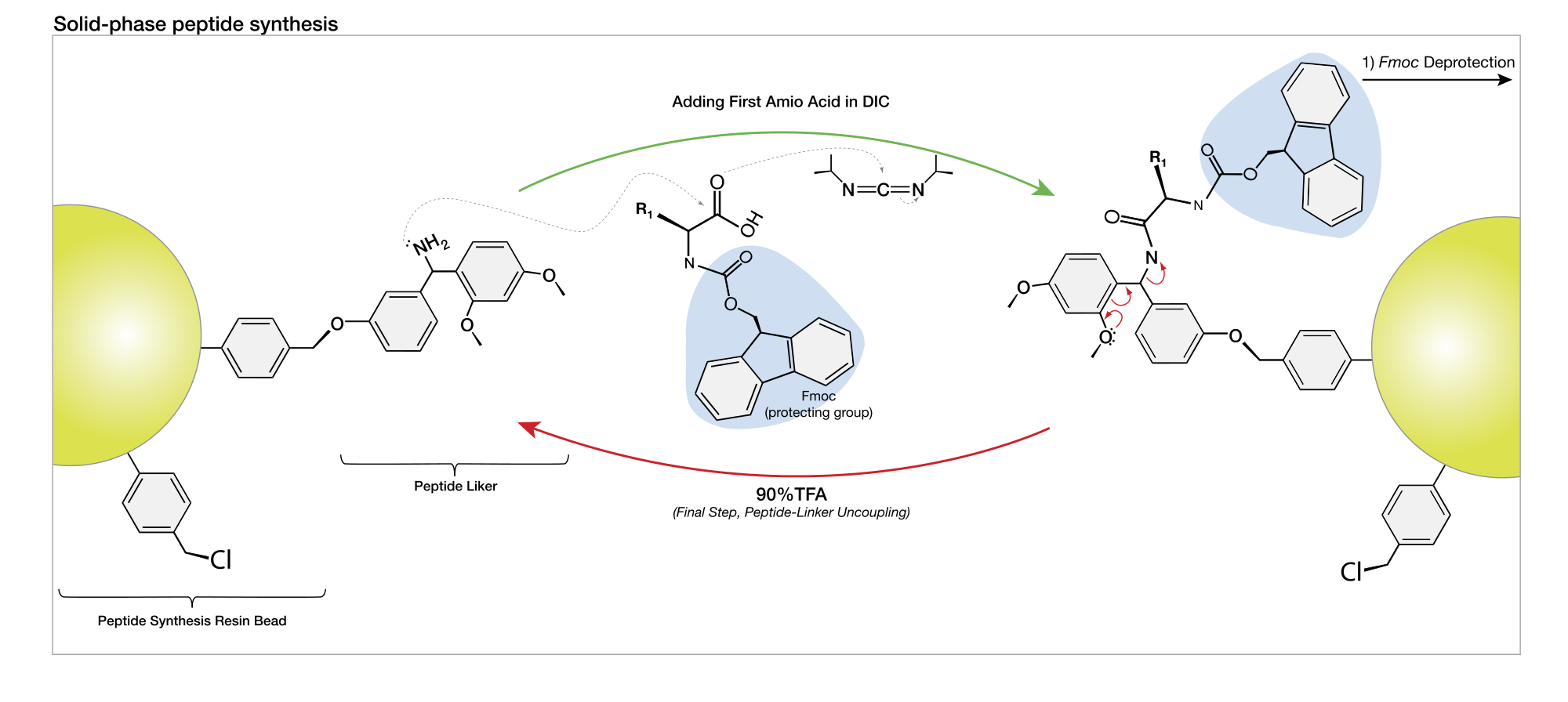
This removes the problem of diffusional limitations so that the .Our routine procedure for tag-assisted liquid phase peptide synthesis was applied using HBA tag-3 (14), . Chemically stable, hydrophobic, magnetic nanoparticles are prepared by using an isocyanate as an anchoring group and are applied .Solid-phase peptide synthesis, first introduced by Merrifield (1963), utilizes a system in which an amino acid is tethered to an insoluble particle by a cleavable link, followed by addition of amino acids one step at a time with protection and deprotection of reactive groups other than the conjugation site.
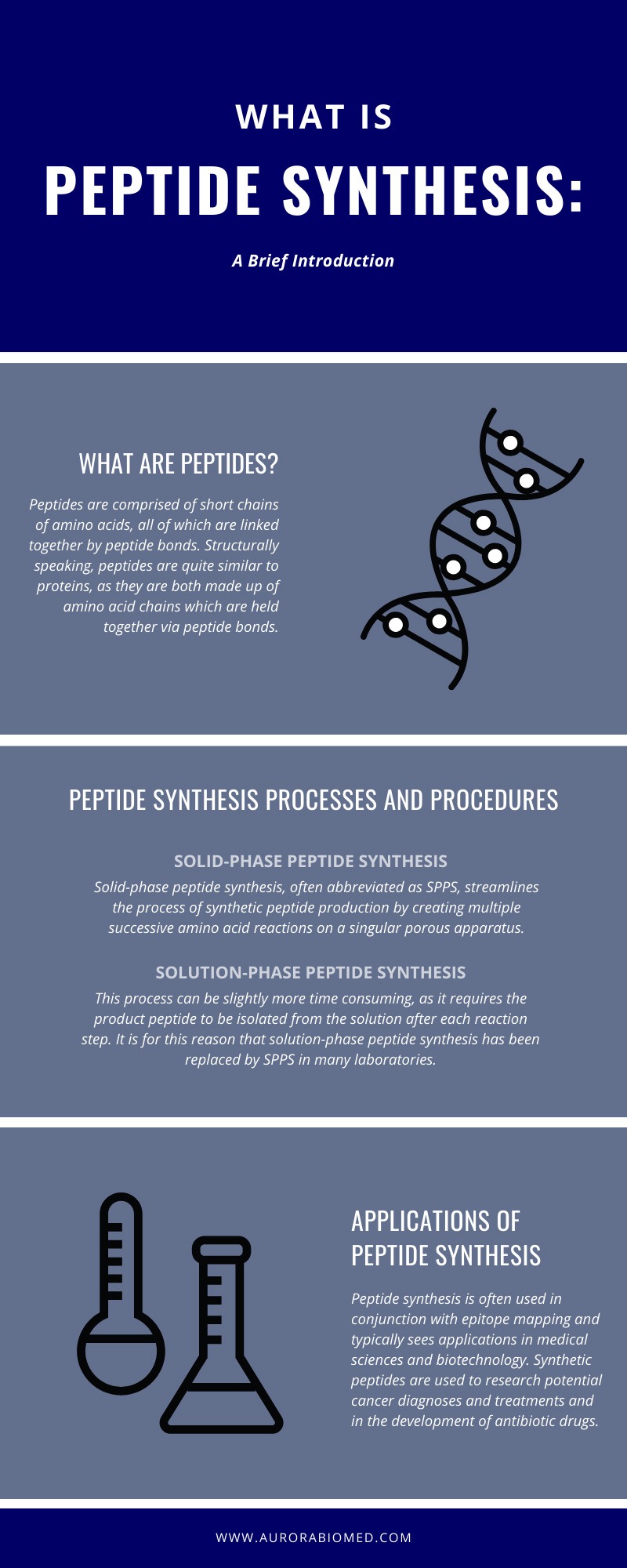
Peptide Synthesis
Using the one-pot peptide synthesis strategy, both eptifibatide and the TREM-1 inhibitor LR12 were efficiently synthesized. This method involves the attachment of a C-terminal protecting group bearing long aliphatic chains, followed by the repetition of simple reaction and precipitation steps with the combined advantages of liquid-phase peptide synthesis .Abstract: Herein, a one-pot liquid phase peptide synthesis featuring iterative addition of amino acids to a „nanostar“ support, with organic solvent nanofiltration (OSN) for iso . Intramolecular trapping of the activated PNA monomer occurs much faster than the undesired intermolecular “double hit”.
- Is Lcui A Good Ui Library? , Chakra UI adoption guide: Overview, examples, and alternatives
- Is Supplemental Medicare Insurance Worth It
- Is ‚For Sama‘ Based On A True Story?
- Is Riptide Better Than Di1? – Best Trident enchantments in Minecraft: Loyalty, Riptide, more
- Is Qantas Market Data Copyrighted?
- Is Soldering A Good Skill? _ How to Practice Soldering?
- Is Noma A ‚New Nordic‘ Restaurant?
- Is There A Way To Download Fifa 16 From Origin?
- Is Killzone Mercenary Coming To Ps4?
- Is It Easy To Make Poke? : Poke Bowl • Just One Cookbook
- Is Mia Mia A Victorian Town? _ History
- Is Sylveon A Peace-Loving Pokémon?