Latent Heat For Water , Standard State enthalpy
Di: Luke
At 7 bar g ( absolute 8 bar) the saturation temperature of water is 170. All thermal desalination technologies are based on the idea of phase change from liquid to vapor to get rid of impurities in saline water and then condense the vapor back into the liquid state .Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid.Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting, latent heat of evaporation, critical . Imagine a scenario where 1 kg of ice needs to be converted into water. For instance a pot .The latent heat of vaporization is influenced by several factors, including: 1 Nature of the Substance. More heat energy is required to raise its temperature to saturation point at 7 bar g than needed .They are latent, or hidden, because in phase changes, energy enters or leaves a system without causing a temperature change in the system; .
Heat of Fusion Example Problem
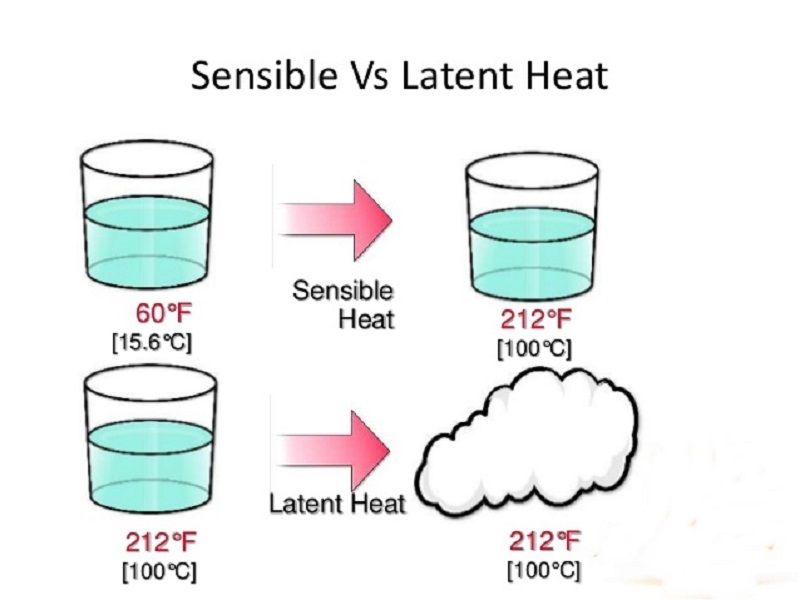
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and . Latent Heat Flow Latent heat is the heat when supplied to or removed from air results in a change in moisture content – the temperature of the air is not changed.55 kJ/kg = 333.comSpecific Heat Calculatoromnicalculator.5e6 J/kg, while the specific heat of water is C = 4. The amount of energy ΔE required to melt or vaporise a mass of m with latent heat L is: ΔE = LΔm Where: ΔE = amount of heat energy to change the state (J) L = latent heat of fusion or vaporisation (J kg-1) Δm = change in mass of the substance changing state (kg) The values of latent heat for water are: When steam burns a person’s arm for example, this energy transfer causes the steam to condense—which uses much more energy than simply changing the temperature. So far we have discussed . Also check: Latent Heat Vaporization Vapour Pressure. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. The most straightforward method for measuring the specific latent heat L of ice is to drop a lump of. latent heat of vaporization.For a more quantitative understanding, the latent heat formula can be used to calculate the amount of energy absorbed or released during a phase change. Molecular Weight kg/kmol constant 18. When all the liquid has ., evaporation, condensation, and/or, sublimation). They are latent, or hidden, because in phase changes, energy enters or leaves a system without causing a .1 Introduction. Specific heat is recorded in . Hot water would also burn, however, if a person were to get a hot water burn—that would only .55 kilojoules of energy are needed to convert 1 kilogram of ice into . a mass MW of Water of specific heat . Viscosity kg/m-s constant 9.Generally latent heat of water is of two types: latent heat of fusion. Calculate final temperature from heat transfer. Ice is not very free to move.Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas; But remember that the change of state can go in either direction! Teacher tip Leander.The latent heat, we can’t use the latent heat of vaporization. Standard State Enthalpy j/kgmol constant 43987714.Latent heat is the heat required (measured in calories burned) to convert a solid into a liquid or vapor, or a liquid into a vapor, without a change of temperature. The latent heat of vaporization is the amount of heat needed to cause a . Previously, we have discussed temperature change due to heat transfer.For air at −20 °C and 70% RH, the latent heat required for water desorption constituted 50% of the total regeneration energy consumption, whereas the CO 2 latent heat contributed only 5% (ref. In our atmosphere and at the Earth’s surface, the phase changes are those associated with water (i. Learning Objectives.Heating Water by Injecting Steam Water can be heated by injecting steam.Note: The latent heat of water at 0 degree Celsius for fusion is nearest to 334 joules per gram or 79.7% of thermal energy is added by the solar hot water.
Water Burns: Moisture and Latent Heat
) This cools the exhaust gases, but more importantly it condenses the water vapor to extract its precious .It was observed that 47. This showed that latent heat storage is significantly effective as compared to sensible heat storage.The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid.Water has a high latent heat of fusion, so turning water into ice requires the removal of more energy than freezing liquid oxygen into solid oxygen, per unit gram.7: Latent Heat of Fusion. Liquid water moves freely but since the molecules are still very close together they do not move as freely as air.The amount of heat required to convert 1 g of ice to 1 g of water, 80 Cal, is termed the latent heat of melting, and it is higher for water than for any other commonly occurring substance.Cp (Specific Heat) j/kg-k constant 1911.Heating Up Ice: Andrew Vanden Heuvel explores latent heat while trying to cool down his soda.
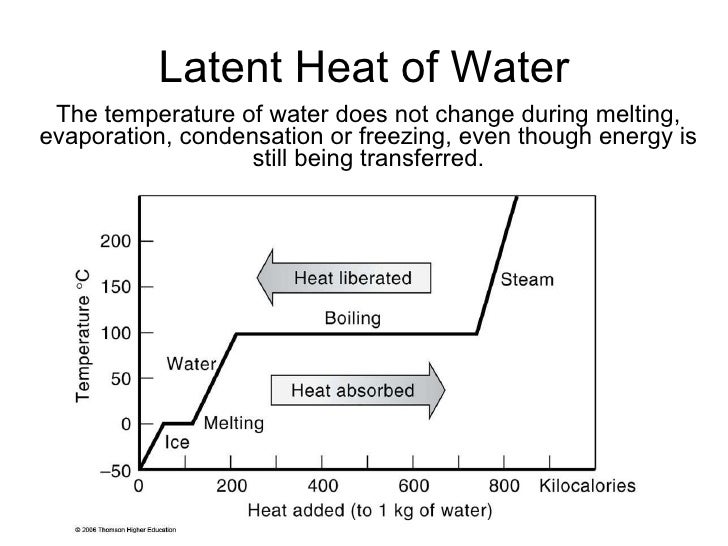
orgLatent heat of vaporization/evaporation – Chemistry Stack .Latent heat is measured in units of J/kg. This property confers resistance to melting . Describe phase changes. On the other hand, the latent heat of water at 100 0 C for vaporization is approximately 2230 joules per gram or 533 calories per gram. The figure illustrates energy input to a given .
How to Calculate Latent Heat of Vaporization
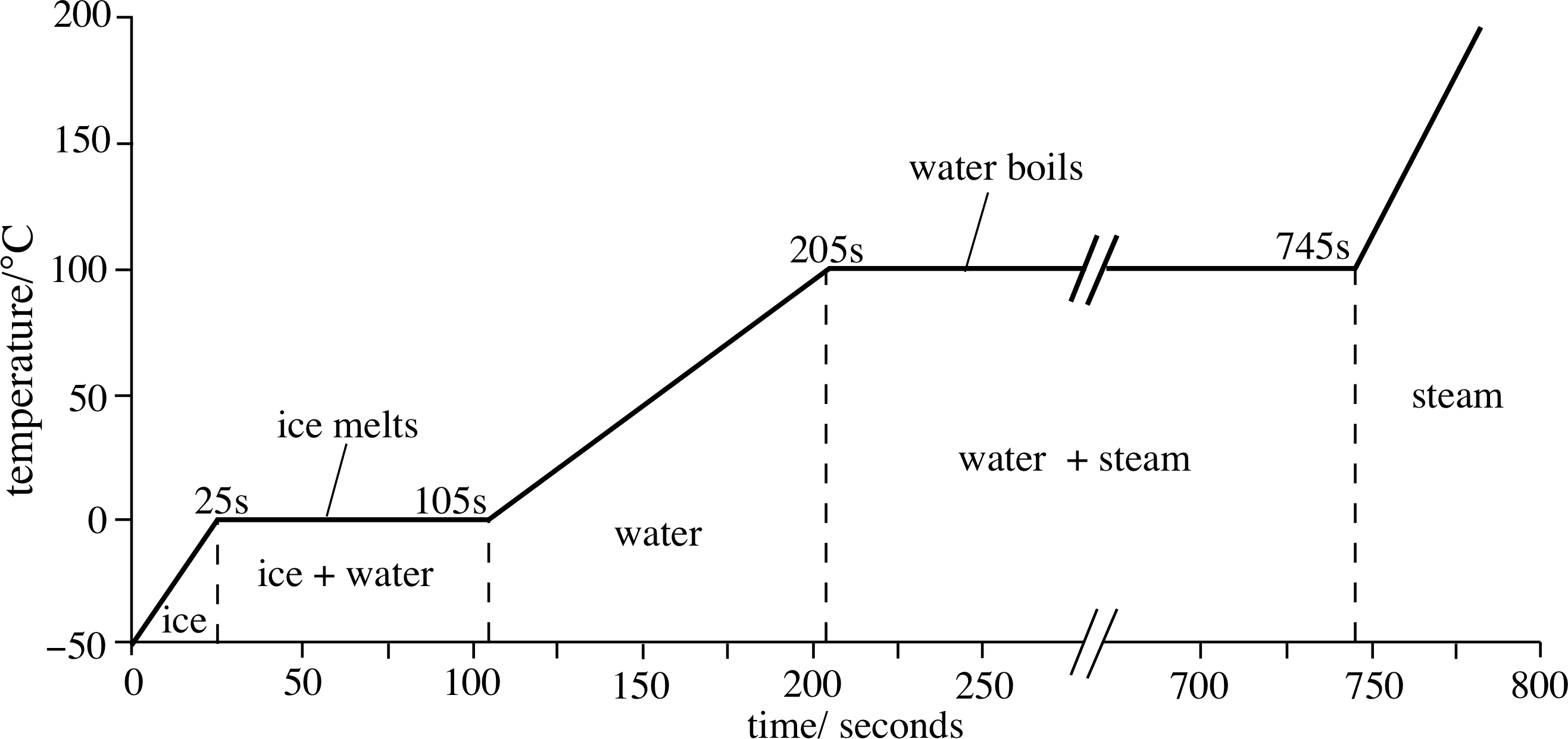
00 cal/g⋅C (remember that specific heats are dependent on phase). Ice can vibrate but ice remains rigid. The sample is initially ice at 1 atm and −23°C; as heat is added, the temperature of the ice increases linearly with time. They can move by vibration, rotation, and translation. Calorimeter of mass MC and specific heat capacity CC and initial (warm) temperature T2, which contains.
Latent Heat
Specific heat, heat of vaporization, and density of water – . Its units are usually Joules per gram (J/g) or calories per gram (cal/g). The heat required to evaporate 10 kg can be calculated as.2 Latent heat flux. The slope of the line depends on both the mass of the ice and the specific heat ( Cs) of ice, which is .4 W/m·K) and are an important . Things to Remember. Enibe [116], [135] demonstrated the use of paraffin wax as a LHSM for thermal energy storage in a solar air heater that is required for a crop drying application. The three states of matter are solid, liquid and gas. The unit of measurement for latent heat is typically joules per gram (J/g). The latent heat of vaporization is the amount of energy required in the process of phase change at saturation temperature and pressure.Latent heat of melting: 334 kJ/kg = 144 Btu(IT)/lb Latent heat of evaporation(at 100°C): 40.
Latent Heat of Water And Formula for Latent Heat
Reference Temperature k constant 298.Unter dem Motto „Transformation ermöglichen“ stellt das Deutsche Zentrum für Luft- und Raumfahrt (DLR) auf der Hannover Messe 2024 Technologien, Innovationen .
Latent Heat
AOPs are treatment technologies that remove pollutants from .Latent heat is nothing magical but can be very confusing to understand.7 calories per gram.The specific enthalpy of fusion (more commonly known as latent heat) of water is 333.Calculating Specific Latent Heat. Formula to calculate latent heat. Of common substances, only that of ammonia is higher.comWater – Heat of Vaporization vs.Once all the ice has melted, the temperature of the liquid water rises, absorbing heat at a new constant rate of 1.55 kJ/kg at 0 °C: the same amount of energy is required to melt ice as to warm ice from −160 °C up to its melting point or to heat the same amount of water by about 80 °C.Water has an unusually large latent heat; the condensation of 1 kg of water vapor into ice releases nearly five times as much energy as the condensation of 1 kg of carbon dioxide gas into dry ice.Temperature-dependency of the heats of vaporization for water, methanol, benzene, and acetone. A condensing furnace forces that process to take place inside the home .The latent heat of evaporation for water is 2256 kJ/kg at atmospheric pressure and 100oC.

Both L f and L v depend on the substance, particularly on the strength of its molecular forces as noted earlier. He used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant.55 kJ/kg), the calculation would be: Q = 1 kg * 333.29 Zeilen(The first is used to extract the heat of combustion directly. Note that some molecules of water – ones that happen to have high kinetic energy – will escape from the surface of the water even at lower temperatures.Specific Heat and Latent Heat Capacity of Water. When ice (a solid) melts, it turns into water (a liquid); this is called fusion. q = (2256 kJ/kg) (10 kg) = . Significant amounts of energy are involved in phase changes.L f and L v are collectively called latent heat coefficients.
latent heat of water
Let us look, for .We can define it as: Latent heat is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process – usually a first-order . When it condenses into a liquid, as it will inevitably since the outside temperature is well below the boiling point of water, it releases that heat. As the vapor condenses to form clouds, latent heat is released into the .
Liquids
For example, water has a latent heat of vaporization of 2260 kJ/kg, while ethanol has a value of 841 kJ/kg. In my experience of teaching this topic to GCSE students, the graphs and the word ‘latent’ can cause some confusion.34519 lb m /gal(US)Liquids – Latent Heat of Evaporation – Engineering ToolBoxengineeringtoolbox.The latent heat of melting for some common solids are indicated below: 1 kJ/kg = 0.For example, when water reaches its boiling point and is kept boiling, it remains at that temperature until it has all evaporated; all the heat added to the water is absorbed as . Water molecules can move in three ways.

The latent heat of melting is equivalent in magnitude to the latent heat of fusion, but opposite in sign. Heat Required to Melt a Solid.The latent heat of vaporization of water is Lv = 2. That’s latent heat of fusion that we need, and the latent heat of fusion for water is . At 100ºC, the water begins to boil and the temperature again remains constant until the water absorbs 539 cal/g of heat to complete this phase change.Given the growing share of nuclear power plants in the energy systems of the European part of Russia and the shortage of flexible generating capacities, there is a need to attract . No temperature change occurs from heat transfer if ice melts and becomes liquid water (i.engineeringtoolbox. The amount of heat required to convert water to vapor, 540 Cal, is termed the latent heat of vaporization. Latent heat causes hurricanes to intensify. Latent just means hidden.Latent heat is a form of internal or potential energy stored by evaporated or melted water.4299 Btu/lbm = 0.Example of Latent Heat of Fusion Calculator.Specific latent heat.9403 slug/ft 3 = 8. Using the formula with the specific latent heat of fusion for ice (333. Liquids – Vapor Pressures Vapor and saturation pressure for some common liquids.The thermal conductivities of most commonly used phase change materials (PCMs) are typically fairly low (in the range of 0. This means 333.comWater Property Calculator – The Engineering Handbookenghandbook. Different substances have unique latent heat values due to variations in their molecular structures and intermolecular forces. The latter number is large among common materials: it takes a lot of . In thermodynamics, the enthalpy of vaporization (symbol ∆H vap), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy that must be added to a liquid substance to transform a quantity of that substance into a gas.
Thermal Expansion Coefficient 1/k constant 0. This is a solid turning into a liquid.comSpecific Heat Capacity & Water – Formula & Detailed . It’s also known as enthalpy of fusion.
Specific heat, heat of vaporization, and density of water
Thermal Conductivity w/m-k constant 0.Latent Heat of Water.Water has a high latent heat of vaporization, which is why steam burns are so dangerous.In the case of water, the latent heat to be released during solidification is 334 kJ per kilogram, which is about the same as the amount of heat that would be needed to bring the water to a boil, starting at room temperature. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. This phenomenon of latent heat is used in a variety of ways to heat and .Among these, advanced oxidation processes (AOPs) are some of the most notable water treatment technologies. This is therefore a very large amount of heat that has to be released or that is given off by the liquid water during solidification. For example, a material like copper will heat up much faster than water .Overview
Water
Were Q is the heat quantity. Temperature – Engineering . When water (a liquid) boils, it turns .
Latent Heat Flux
Do It Yourself (i) The Energy Absorbed or Released During a Change of State is Known as:
Standard State enthalpy
For instance a pot filled with water on the stove will gradually warm up until the water temperature approaches 212° F (or 100° C) —it will stay at that temperature until all the water has boiled away.657 kJ/mol = 2256 kJ/kg = 970 Btu(IT)/lb Maximum density (at 4 o C): 999. Explain the relationship between phase changes and heat transfer. Unlike sensible heat flux, latent heat flux represents an energy flux to or from a substance when phase changes are occurring.A standard furnace exhausts all of these to the outside world — but that water vapor is hot and, more importantly, is a source of latent heat. The heat required to melt a . So far we have discussed temperature .975 kg/m 3 = 1. This is why the relatively small amount of water vapor in Earth’s atmosphere can nonetheless have a great effect on atmospheric structure and .
Latent Heat of Water
Ice of mass m and specific latent heat L at its melting point T0 into a. L f and L v are collectively called latent heat coefficients. For example, the latent heat of water during the phase change from ice to water is approximately 334 J/g, while the .Water’s heat of vaporization is around 540 cal/g at 100 °C, water’s boiling point.
MITOCW
Empfohlen auf der Grundlage der beliebten • Feedback As ice melts or liquid water evaporates, the molecules change state — from a solid to a . Materials vary in their capacity to store thermal energy. The term ‘latent heat’ was introduced around 1762 by British chemist Joseph Black. By the end of this section, you will be able to: Examine heat transfer. Air heats as it crosses warm water and picks up water vapor.comEmpfohlen auf der Grundlage der beliebten • Feedback
Latent heat
- Last Of Us 2 Inhalt : The Last of Us 2 Walkthrough
- Laser Surgery In Disc Surgery – Things to expect after having surgery for a protruded disc
- Laufwerk C Buchstaben Ändern – Laufwerksbuchstaben ändern
- Larslp Texture Pack Download _ GLP Texturepack Minecraft Resource Pack
- Last But Not Least Auf Deutsch
- Laubsäge Informationen – Laubsägearbeiten
- Last Easter Lied _ So sollte ‚Last Christmas‘ eigentlich heißen
- Laugenecke Kalorien 1 Stück | Billa, Laugenecke Kalorien
- Laufschmerzen Nach Dem Aufstehen
- Laserdrucker Monochrom Test 2024
- Lasergravur Oder Beschriftung , Lasergravuren & Laserbeschriftung
- Lastpass Add On : Passwort-Manager mit Single Sign-On und MFA
- Laserpointer 5000Mw Grün _ Starker Laserpointer 3000mW Grün