Lattice Energy Calculation _ Optimize Your Calculations with the Lattice Energy Calculator
Di: Luke
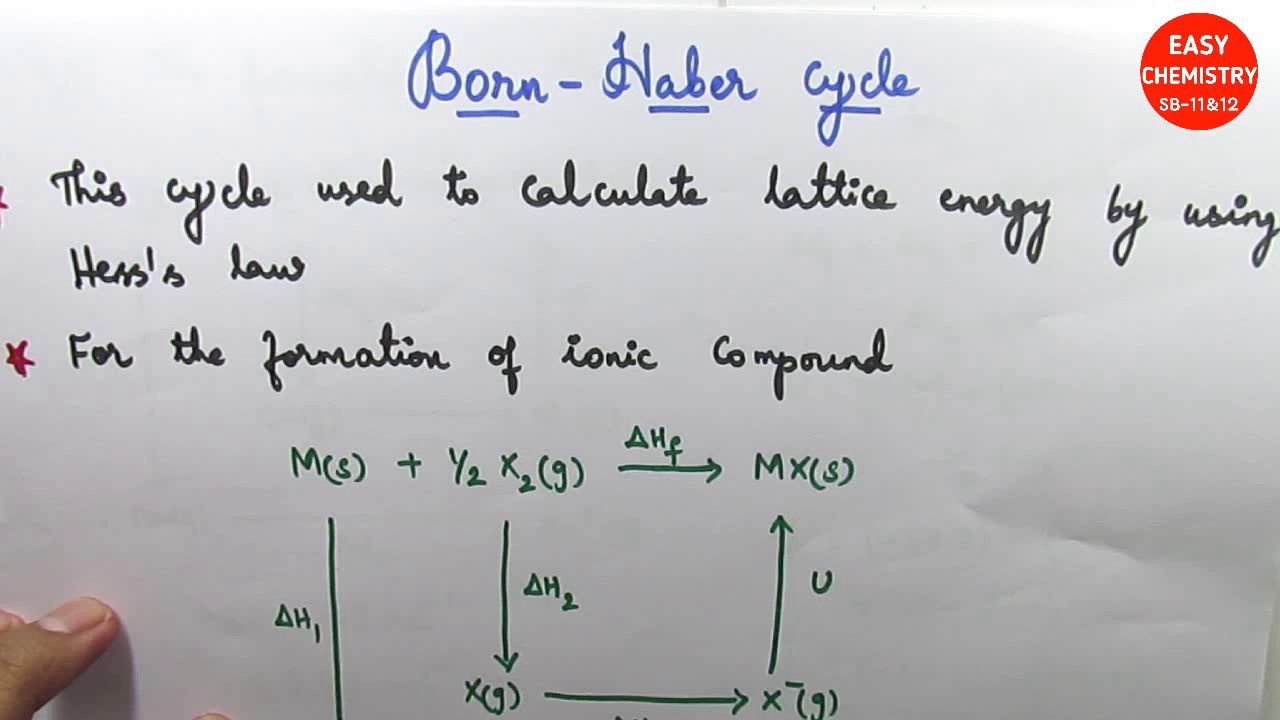
The lattice energy (\(U\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; \(U\) can be calculated from the charges on the .1: Lattice Energies.0 license and was authored, remixed, and/or curated by Patrick Fleming.Born Haber Cycle, Basic Introduction, Lattice Energy, Hess .
Lattice Energy Calculator
And no – I am not being careless about this! Calculations of this sort end up with values of lattice energy, and not lattice enthalpy. MaLb(s) → aMb+(g) + bXa−(g) (1) (1) M a L b ( s) → a M b .Born-Haber Cycle.Methods for Calculating Lattice Energy. Lattice energy, based on the equation from above, is dependent on multiple factors.There are other factors to consider for the evaluation of lattice energy and the treatment by Max Born and Alfred Lande led to the formula for the evaluation of lattice energy for a mole of crystalline solid. Example \(\PageIndex{1}\) Which compound has the greatest lattice energy? AlF 3; .The lattice energies and the lattice self-potentials at many lattice sites were calculated for a series of perovskites. [2] Some chemistry textbooks [3] as well as the widely used CRC Handbook of Chemistry and Physics [4] define lattice .6}\)) is a means of calculating the lattice energy of a crystalline .
What is Lattice Energy? How to Calculate Lattice Energy?
1: Lattice Energies – Chemistry LibreTextschem.Much more should be considered in order to evaluate the lattice energy accurately, but the above calculation leads you to a good start. Expand/collapse global location. Schematic representation of lattice energy at inter-ionic distance r o.Calculation of lattice energy. Born-Haber Cycle.Lattice energy refers to the energy which is released while two oppositely charged gaseous ions attract to each other and form an ionic solid.Lattice Energy Calculator – University of Sydneyscilearn.
Explain the formation of cations, anions, and ionic compounds.48 kJ/mol; Lattice Energy= [-641. R is the distance between ions.learntocalculate.
Lattice energy
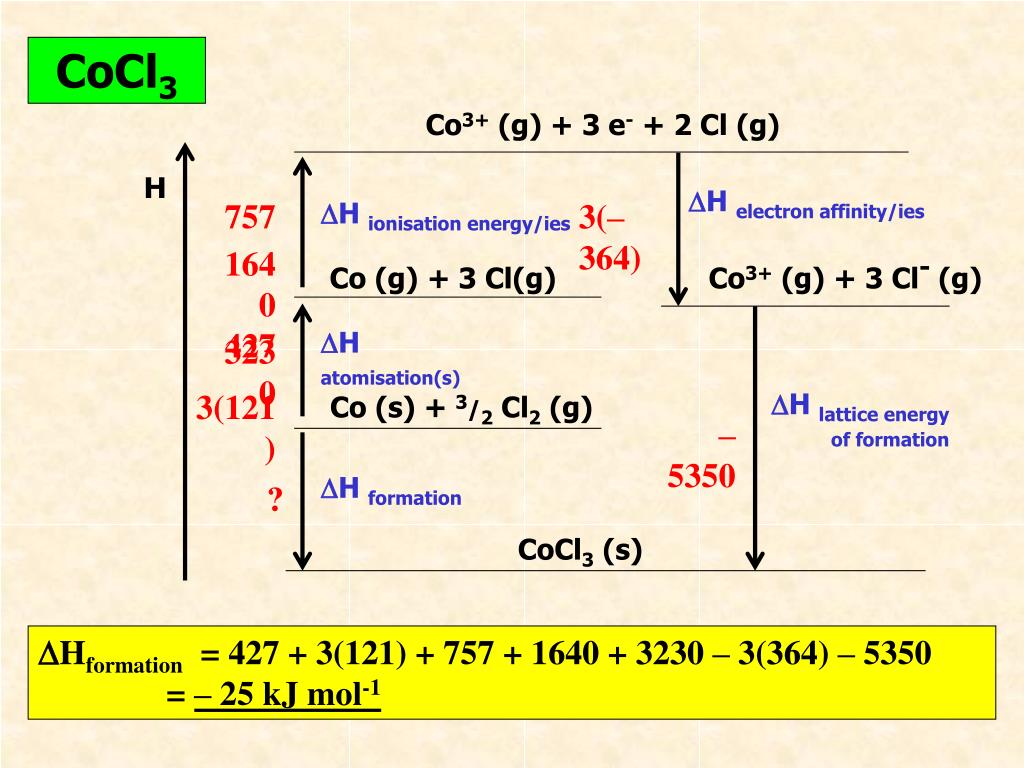
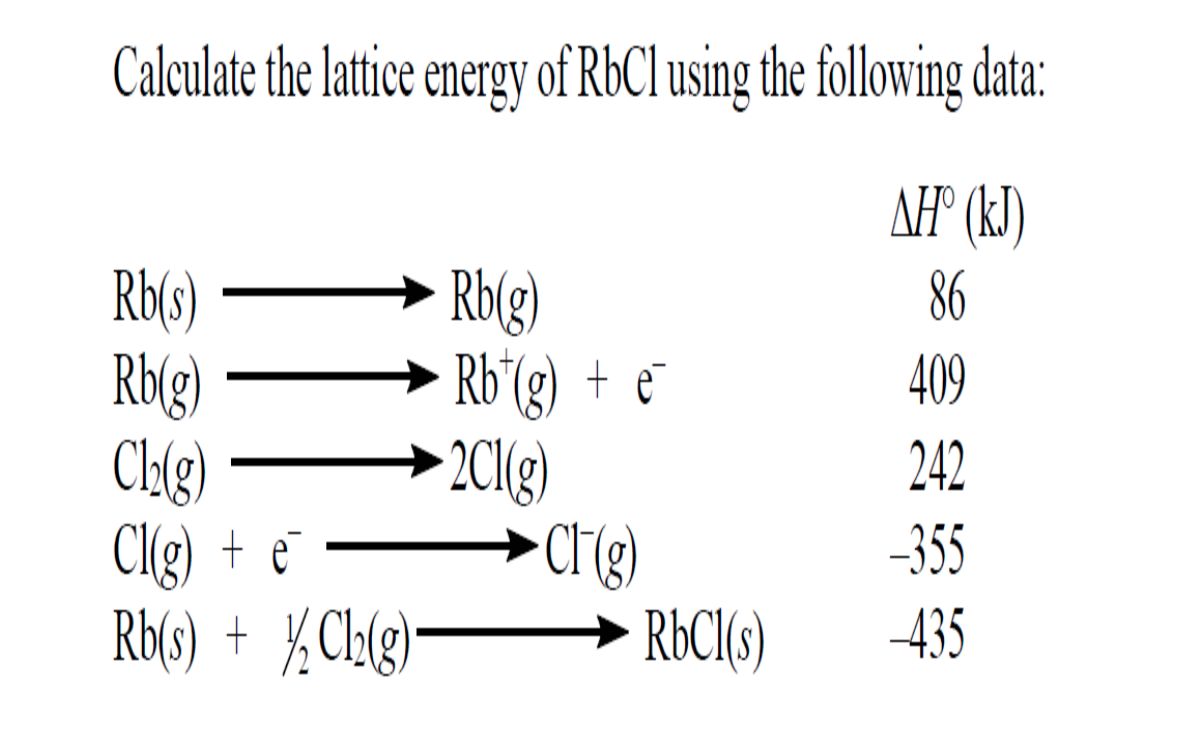
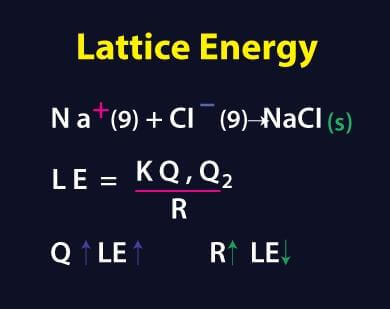
The calculation of Gibbs free energy of intermolecular interactions, also known as lattice energy, is one of the biggest challenges in computational chemistry and . The negative sign of the energy is indicative of an exothermic reaction. This technique . The first step is to construct a cluster of sufficient size around a molecule, typically with .Calculate Lattice Energy.auHow To Calculate the Lattice Energy? – Easy to Calculateeasytocalculate. M +(g) + X –(g) → M + X –(s) +Energy . Therefore, when used in calculating the lattice energy, we must remember to subtract the electron affinity, not add it.Learn how to estimate lattice energy using Coulomb’s law and the charges and radii of ions. Chemical Physics . Even so, it can be . (7) derived by us and compared with the results of theoretical methods commonly encountered in the literature and experimental values determined from Born-Fajans-Haber thermodynamic cycle the computed lattice energy values. Find the Lattice Energy of NaCl (s) by the Born-Haber cycle method.Lattice energy is a calculation of ionic bond strength in an ionic compound. Charge of Cation – .7: Lattice Energy and the Born-Haber Cycle is shared under a CC BY-NC-SA 4.The Lattice energy, U U, is the amount of energy required to separate a mole of the solid (s) into a gas (g) of its ions. Z + and Z – = Cation and anion charge. Also, it can be described as a method of measuring cohesive forces that bind ions. For certain ionic compounds, the calculation of the lattice energy requires the explicit inclusion of polarization effects.In 1918 Max Born and Alfred Landé proposed that the lattice energy could be derived from the electrostatic potential of the ionic lattice and a repulsive potential energy term. In an Ionic solid, lattice energy cannot be directly measured. Let us take a closer look at these methods.In this example, we calculate the lattice energy of lithium oxide (Li2O) using the Born-Haber cycle and Hess’s Law. Lattice energy is defined as the energy released in the .The formula for calculating lattice energy is as follows: LatticeEnergy=dk×∣Q1×Q2∣. Table 3 contains lattice .

The Relationship between Lattice Energies and Physical Properties.Lattice energy calculation – A quick tool for screening of cocrystals and estimation of relative solubility.Usually, energy released would have a negative value, but due to the definition of electron affinity, it is written as a positive value in most tables. α = Madelung constant.The fundamental formula powering the Lattice Energy Calculator is expressed as follows: U = – (k * Q1 * Q2) / r. The formation of a mole of ionic solid from the constituent gaseous ions may be represented by “U”. Calculations of this sort end up with values of lattice energy, and not lattice enthalpy.Initially, we calculated the lattice energy values for alkali halides using Eq. Determine the lattice energy for NaCl by using the Born-Lande . Note the Pattern.comLattice energy (video) | Khan Academykhanacademy.Mike Blaber ( Florida State University) Modified by Joshua Halpern ( Howard University) 9. There are multiple ways to calculate lattice energy, including the Born-Haber cycle and the Kapustinskii equation. Lattice energies of molecular crystals can also be obtained with CrystalExplorer17, using the same CE-B3LYP interaction energies that underpin the calculation of energy frameworks. For sodium chloride, the lattice energy, U, is equal to the enthalpy change for the reaction. Using four different benchmark sets of molecular crystals, we have established the level of confidence for lattice energies estimated using .orgEmpfohlen basierend auf dem, was zu diesem Thema beliebt ist • Feedback
Lattice Energy: The Born-Haber cycle
Q2 is the numerical ion charge of ion 2. n = Born Exponent r 0 = Closest ion distance. Therefore, the lattice energy is 8.with ΔHLat = 672kJ / mol. It gives insights into various characteristics, including its volatility, solubility, and durability of ionic solids.Using a lattice energy calculator involves entering the relevant parameters, such as ion charges and distances, into the tool. Lattice dissociation enthalpies are always . When methods to evaluate the energy of crystallization or lattice energy lead to reliable values, these values can be used in the Born-Haber cycle to evaluate other chemical properties, for example the electron affinity, .The size of the lattice energy is connected to many other physical properties including solubility, hardness, and volatility. There are other factors to consider for the evaluation of lattice energy and the treatment by Max Born and Alfred Lande led to the formula for the evaluation of lattice energy for a mole of crystalline solid.Rearrangement to solve for lattice energy gives the equation: Lattice energy= Heat of formation- Heat of atomization- Dissociation energy- (sum of Ionization energies)- .comEmpfohlen basierend auf dem, was zu diesem Thema beliebt ist • Feedback
Lattice Energy Calculator
This is the energy necessary to take one mole of a crystalline solid to ions in the gas phase.Lattice Energies.Calculating Lattice Energies: Only one unique molecule in the unit cell – Z‘ = 1 Z ′ = 1. Lattice energy is the amount of energy released when. In these cases the polarization energy E pol associated with ions on polar lattice sites may be included in .
Lattice Energy using Original Kapustinskii equation Calculator
Lattice Energy= [-436. This calculator should be used in conjunction with the notes on ‚Understanding Crystal Structures‘ . The calculator then performs the necessary mathematical operations to provide the lattice energy value. Watch a video and see examples and explanations of lattice energy calculations . Dive into the realm of crystal energy . Similarly, as the distance between ions decreases, the lattice energy increases as the ions come closer together, resulting . This page titled 3.

Lattice Energy; Calculation of Lattice energy
2: Ionic Bonding and Lattice Energy is shared under a CC BY-NC-SA 4. These results indicated that the stability of the .Lattice Energy Definition, Trend, Formula, And Lattice .By doing physics-style calculations, it is possible to calculate a theoretical value for what you would expect the lattice energy to be.orgHow to Calculate Lattice Energy. This user-friendly approach empowers individuals to focus on the conceptual aspects of lattice energy rather than .6}\)) is a means of calculating the lattice energy of a crystalline ionic compound and derived from the .6022 × 10-19 C). and subtracting from them the ground state energy of LiH(s . If you know how to do it, you can then fairly easily convert between the two.The lattice energy is defined as the energy required to bring about the following process, LiH(s) —-> Li + (g) + H-(g) The determination of the lattice energy on the basis of the proposed model, therefore, proceeds by calculating the ground state energies of Li + (g) and H-(g).Na + (g) + Cl − (g) → NaCl (s) which amounts to −786 kJ/mol.How lattice energy can be calculated? What is the lattice energy of CaCl2? What is the lattice energy of MgO? What is the lattice energy of KCl? The .(Last Updated On: 2024-03-07) Discover the power of Newtum’s Lattice Energy Calculator, a tool designed to simplify complex calculations.Learn how to calculate lattice energy using different methods, such as experimental, Born-Haber cycle, hard-sphere model, and ionic model. Number of Ions – The Number of Ions is the number of ions formed from one formula unit of the substance.
The total potential energy . Where: As the charges of the ions increase, the lattice energy also increases due to stronger electrostatic attraction. U L = equilibrium value of the lattice energy.The Born–Landé equation (Equation \(\ref{21.
Lattice Energy; Calculation of Lattice energy
There are several different equations, of various degrees of . According to this . Case of flavonoids – ScienceDirect.
Optimize Your Calculations with the Lattice Energy Calculator
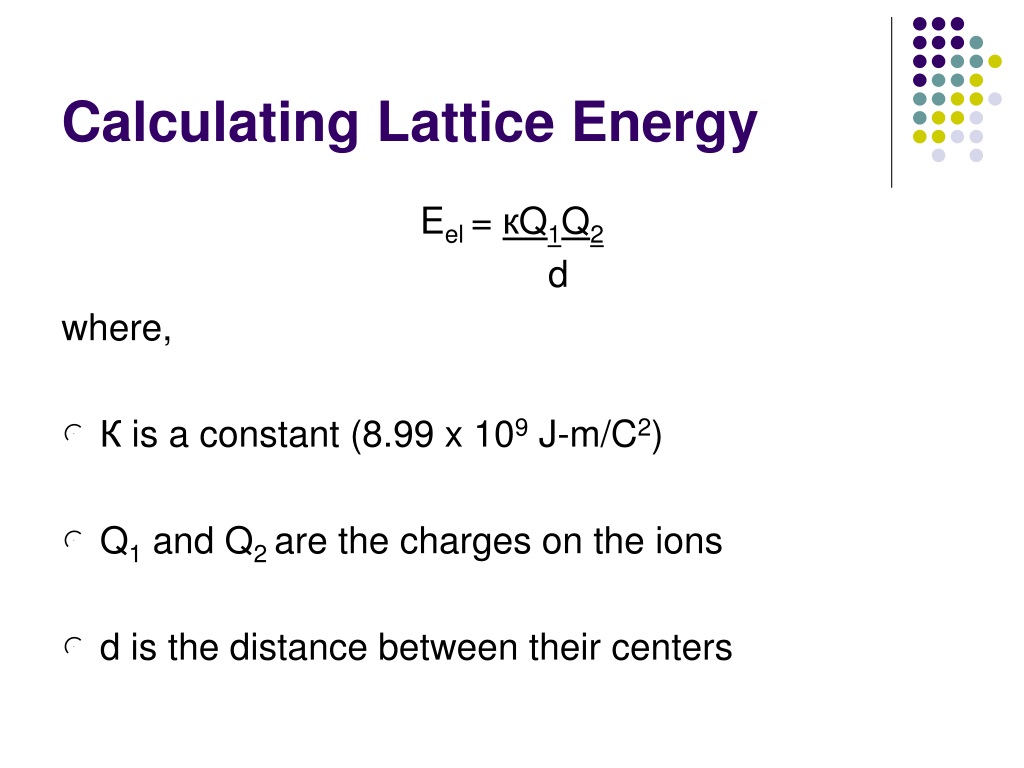
= + where: N A = Avogadro constant;; M = Madelung constant, relating to the . k: Coulomb’s . In general, electron affinity increases from left to right across the . In addition, as ions come into closer contact, lattice energy also increases. MaLb(s) → aMb+(g) + bXa−(g) (7. Example: Na Cl has an ion charge of +1 and -1, calculate the lattice energy if the distance between the two ions is 0.8-146-243-(737. The calculation of lattice energy can be done by using Hess’s law (for this case it is called Born Haber cycle). ϵ o = Permittivity of free space. There are several important concept to understand before the Born-Haber Cycle can be applied to determine the lattice energy of an ionic solid; ionization . The Born-Haber cycle is a thermochemical method that uses Hess’s law to calculate lattice energy indirectly. First Year Chemistry in the School of Chemistry at .0 license and was authored, remixed, and/or curated by LibreTexts.Calculating Lattice Energies.The Born–Landé equation is a means of calculating the lattice energy of a crystalline ionic compound. Enter numbers and get the lattice energy of any ionic compound in kJ/mol.3: Lattice Energies in Ionic Solids – Chemistry LibreTextschem.The lattice energy (U) of a crystal is the energy that evolved when one gram of the crystal is formed from gaseous ions. Skills to Develop. Solved Examples.Lattice Energy Calculator.
N A = Avogadro’s constant (6. The amount of energy needed to separate a gaseous ion pair is its bond energy. Example 1: Compute the Lattice energy of NaCl by using Born-Lande .
An important enthalpy change is the lattice energy. The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions.Lattice energy is defined as the amount of energy released when cations and anions are brought from infinity to their respective lattice side in a crystal to form one mole of the ionic solid. Where: U: Lattice energy. We see that the charge of ions is proportional to the increase in lattice energy.8-(-328)] kJ/mol= -695.
ukEmpfohlen basierend auf dem, was zu diesem Thema beliebt ist • Feedback
Lattice Energy
19 x 10^-19kJ/mol.Lattice Energy for Kapustinskii Equation – (Measured in Joule per Mole) – Lattice energy for Kapustinskii Equation of a crystalline solid is a measure of the energy released when ions are combined to make a compound.6)-(2*-349)] kJ/mol= -2521.
How to calculate lattice energy
chemdictionary.Born–Landé Equation.This chemistry video tutorial provides a basic introduction into the lattice energy of ionic compounds.comlattice enthalpy (lattice energy) – chemguidechemguide. e = Electron charge (1.
- Laterne Weiß Outdoor : Laterne für draußen wetterfest, Weiß kaufen
- Lauinger Hamm Rehaklinik , Ernährungsberatung für zu Hause
- Laserdrucker Adf | Verstehe was ADF beim Drucker bedeutet
- Lavendel Im Halbschatten : Lavendel: Pflanzen und pflegen
- Last Easter Lied _ So sollte ‚Last Christmas‘ eigentlich heißen
- Laufwerk Funktioniert Nicht Mehr
- Lässig Krankenkasse _ „Arbeitslos und lange krank: Welche Leistungen stehen mir zu?“
- Last Chaos Dropliste Waffen _ EX / Rogue weapons
- Laufende Zinsen Tabelle : Zinseszinsrechner: Zinsen einfach berechnen
- Lavendel Heilwirkungen | Lavendel
- Laser Tag Weiden – Lasertag in Weiden in der Oberpfalz