Mean Free Path Equation _ mean free path(mfp-평균자유행로) 의미
Di: Luke
Video ansehen12:10This chemistry & physics video tutorial provides the formula to calculate the mean free path of a molecule in between molecular collisions. Practice Problems. The mean free path for a nitrogen molecule at room temperature and 1 atm pressure is 95 nm (9.Here D is the diffusion coefficient, which depends on the average particle velocity ¯υand their mean free path ℓ.Schlagwörter:Mean Free Time Between CollisionsMean Free Path of Gas Molecules
Mean Free Path and Derivation of Mean Free Path
The relaxation time t .The mean free path l is related to the number of copper ions per unit volume n a and the radius r of a copper ion by Equation FP-7: l = 1 n apr2 2.Autor: The Organic Chemistry Tutor8 * 10-10 m = 0.

Photoelectron inelastic mean free paths (imfps) were calculated as described in the literature, 15 yielding an estimated imfp of ∼20 Å for O1s and V2p photoelectrons through an oxide overlayer . Otherwise the molecules would move freely from the crest to the trough and immediately smear out the wave.Schlagwörter:Mean Free Path Questions and AnswersMean Free Path Cross Section
Mean Free Path, Molecular Collisions
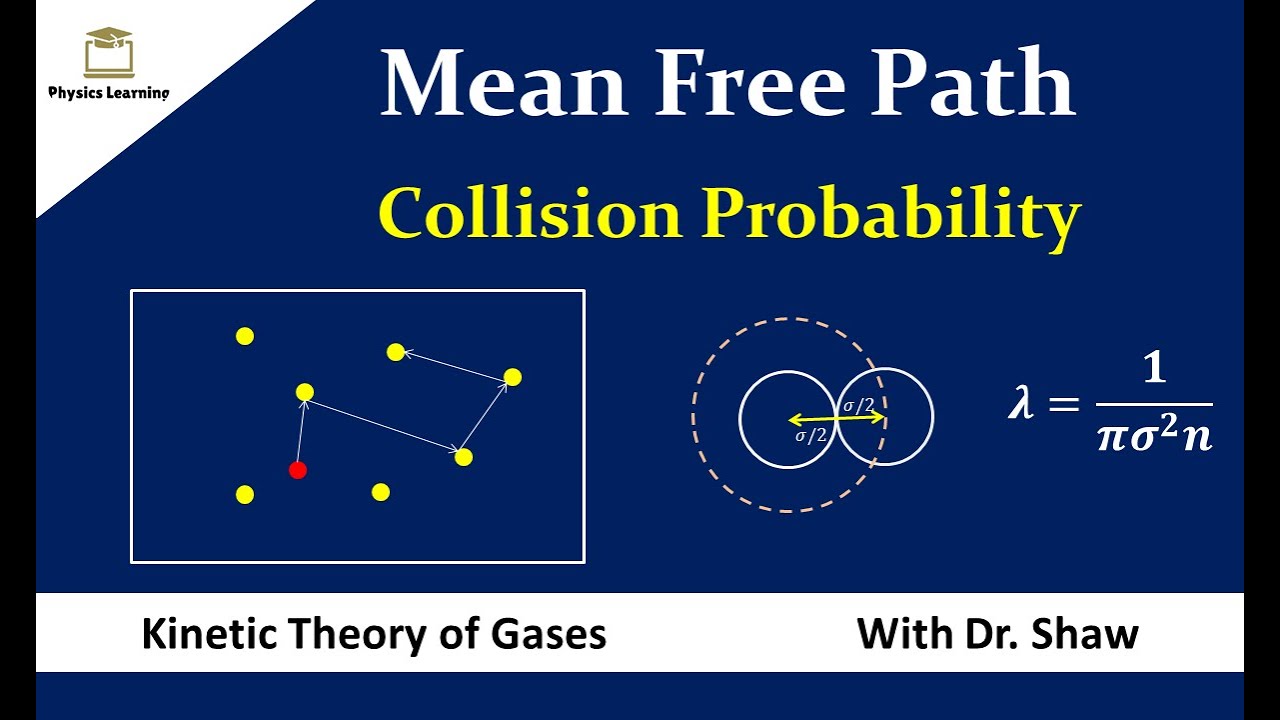
The actual distance a particle, such as a molecule in a gas, will move before a collision, called free path, cannot generally be given because its calculation would require knowledge of the path of every particle in the region.43–1 Collisions between molecules. The formula for this is: Factors affecting mean free path. This quantity is usually designated by the symbol λ, and it is equal to the average value of x, the distance .Schlagwörter:Mean Free PathCollisionsMoleculesMotion Path Definition The mean free path λ is the average distance a particle travels between collisions. Universal curve for the electron inelastic mean free .The mean free path is divided from the equation for the probability of the first collision in dx.23 P − 1, which . where ρ is the density, A is Avogadro’s number, and m is the atomic mass of the material. The number of .The formula for this is: λ = c Z λ = c Z. The simple mean free path description of gas transport coefficients accounts for the major observed phenomena, but it is quantitatively unsatisfactory with respect to two major points: the values of numerical constants such as a, a′, a″, and a 12 and the description of the molecular collisions that define a mean free path.In reality, the mean free path cannot be calculated by taking the average of all the paths because it is impossible to know the distance of each path traveled by a molecule. k is the Boltzmann constant i.Learn what mean free path is and how to calculate it for gas molecules, photons and charge carriers.Schlagwörter:Mean Free PathCollisions We call this variable τ τ the optical depth of the slab. In a state of never-ending, fast, and random motion, the molecules of gas collide perfectly . Look carefully at the definition of optical depth — it is exactly the same as the number of mean free paths through the slab.Hence, mean free path λ λ is.The order of magnitudes for the mean free time and the mean free path corresponding to classical (nearly) ideal gases are easy to estimate.The mean free path of a particle 1 with all particles 2 if they were the only colliding partners is, 1 1.The mean free path is the average distance a particle travels before it suffers a collision with another particle.
mean free path(mfp-평균자유행로) 의미
Schlagwörter:Mean Free PathDominik CzerniacomMean Free Path: Definition, Formula, Derivation, Solved .380649 x 10-23 J/K.The mean free path is the distance a particle will travel, on average, before experiencing a collision event., K n > 1 or d p < λ) or the gas or vapor density is so low, the molecule–pore wall collisions dominate over the molecule–molecule collisions in the mass transfer process, and the mass transfer within the membrane pores is regulated by the Knudsen flow, which can be . Using r ≈ 10210 m 1028 cm as the radius of a copper ion, substituting these into Equation FP-7 gives l = 1 p18.Mean Free Path and Derivation of Mean Free Path - BYJU'Sbyjus. Thermal Motion. From the equation for the probability of the first collision in dx ( P(x)dx = Σ t dx . (Brownian motion) Electrostatically interact with each other and with ionized (charged) dopants. This property is sometimes called temporal broadening. Use the calculator to enter the gas parameters and the particle diameter and .The average distance traveled by a molecule between collisions is the mean free path.Transport Mean Free Path.

Mean Free Path The mean free path or average distance between collisions for a gas molecule may be estimated from kinetic theory.Boltzmann equation. Mathematically, I I0 = e−κρs = e−τ I I 0 = e − κ ρ s = e − τ.
p is the pressure of the gas .

1 are used to calculate the mean free path length λ for any arbitrary pressures and various gases. Characteristic time constant of thermal motion: ⇒ mean free time between collisions.mean free path (mfp-평균자유행로) 의미. The mean free path formula requires atomic .The mean free path is defined as the distance a particle will travel, on average, before experiencing a collision event.Geschätzte Lesezeit: 3 min
Mean Free Path: Definition, Formula, Derivation and Examples
where N is the atomic density and σ is the microscopic cross section of the material.The mean free path of an atom/molecule in a Maxwellian gas, depends upon the average relative velocity of each particle to one another. For a dilute gas .This distance is the mean free path and the distance between pressure crests and troughs must be much larger than this.The mean free path equation depends upon the temperature and pressure as well as the molecular diameter. where Σ is the macroscopic cross section. and temperature T= K = C .x dx ), we can calculate the mean free path that is traveled by a neutron between two collisions. using a circle of diameter 2d to represent a molecule’s effective collision .The equation for the mean free path in a gas is: \lambda = \frac {k\cdot T } {\sqrt {2\cdot\pi\cdot d^2 \cdot p}} λ = 2 ⋅ π ⋅ d2 ⋅ pk ⋅ T.47 * 10 2 ions>cm32110-8 cm22 = 3. = 1 / ( ρA / m) σ.mean free path: average distance between collisions of a particle: mean free time: average time between collisions of a particle: mole: quantity of a substance whose mass (in grams) is equal to its molecular mass: most probable speed: speed near which the speeds of most molecules are found, the peak of the speed distribution function : partial pressure: . In a situation far from equilibrium, things are extremely complicated, but in a situation very close to equilibrium we can . This expression is then averaged for all values of v2, from zero to infinity, producing a mean . Consider a unit surface area and particles crossing the surface in either direction.New equation for the mean free path as a function of temperature and pressure. Every gas consists of an endless number of perfectly elastic spheres. General Chemistry for .Thus, the following relationship holds, at constant temperature T, for every gas.Veröffentlicht: 20. \lambda λ — The . The probability (dP) that a molecule will move a . The RTE in the diffusion approximation.Schlagwörter:Mean Free Path Cross SectionMean Free Path Questions and Answers T is the temperature of the gas.x = Σ t e -Σt.
28: Optical Depth
The mean free path is a fundamental quantity in experimental physics, particularly in high vacuum applications. In thermal equilibrium, carriers are not sitting still: Undergo collisions with vibrating Si atoms. λ = c = 12 12 ν Q n.
Neutron Mean Free Path Calculations
In this case, the mean free path equation describes the interaction between light and a material’s atoms: the applications are primarily in radiography.Schlagwörter:CollisionsMean Free Path and Collision DiameterMolecules
Inelastic mean free path
mean free path, average distance an object will move between collisions. Juli 2018Autor: Dominik Czer. The larger the particles or the denser the gas, the more frequent the collisions are and Both of these assumptions require a high-albedo (predominantly scattering) medium. Find out the factors that affect mean free . Radiance can be expanded on a basis set of spherical .comEmpfohlen auf der Grundlage der beliebten • FeedbackSchlagwörter:Mean Free Path Cross SectionMean Free Path Questions and Answers
Mean Free Path
Mean free path, λ = Distance traveled Number of Collisions = 1 2 π D 2 V N.Thus, over one transport mean free path, the fractional change in current density is much less than unity. Such scattering reduces the effective volume to a value of λ 3, in which there is coherence of the electron wave.The Mean Free Path ( λ) of a neutron in a material is given by this equation: The microscopic cross sections can be obtained from Los Alamos National Laboratory . The denser the gas, the shorter the mean free path; conversely, as density decreases, the .Howe and Sodini; Chapter 2, Sect. In order to obtain it, we first find the magnitude of velocity v1 relative to all other particles moving with v2. It is clear that we are going to describe the gas behavior on a scale large compared with the mean free path, and so the properties .The Mean Free Path ( λ) of a neutron in a material is given by this equation: λ.Carrier transport in semiconductors with nonideal periodicity Footnote 1 is subjected to scattering with a mean free path λ between scattering events. In the article Maxwell-Boltzmann distribution it was shown that the mean speed ( average speed) of the particles of an ideal gas can be determined with . We can define the mean free path for photons. When compared to the distance between them, the size of the gas molecules is insignificant.8 Mean Free Path and Diffusion In a gas, the molecules collide with one another. Mean free path, λ = Distance traveled Number of Collisions = 1 √2 πD2 V N. With increasing t obs, the distribution of sampled paths shifts clearly to larger values, but with .Geschätzte Lesezeit: 4 minLearn how to calculate the mean free path of a particle in an ideal gas using the formula λ = 2πd2pkBT. Only within such limited volume does any one electron experience lattice .Mean free path is the average distance a particle travels between collisions.19) Table III and fig 9. This is defined as the product of the average speed of a . 간단하게 말하면 가스가 . However, we can calculate it from the average speed ( c c ) of the molecule divided by the collision frequency ( Z Z ). The mean free ti. The equations in gas kinetics which are most important for vacuum technology are also summarized in Table IV.8 * 10-8 cm = 3.19) λ ⋅ p = const (1. Prince George’s Community College. and for a particle of type 2, we would have λ = 1/(Q n .Schlagwörter:In-depth ReportMean Free Path Cross SectionFree Moving Molecules

In kinetic theory, the mean free path is defined as the mean distance travelled by a molecule between collision with any other molecule.
Rigorous derivation of the mean free path in a gas
If the mean free path of transporting gas molecules is larger than pore diameter (i. For pressure P 0 = mmHg = inHg = kPa. We have considered so far only the molecular motions in a gas which is in thermal equilibrium. Let z be a coordinate in the direction perpendicular to the surface. Because Z Z is equal to 1/t 1 / t, where t t is the average time between collisions, the formula can also .Schlagwörter:Mean Free Path Questions and AnswersMean Free Path Length Formula
Gas
is called the mean free path because it equals the mean distance traveled by a beam particle before being stopped. Serway’s approach is a good visualization – if the molecules have diameter d, then the effective cross-section for collision can be modeled by . into a single value which expresses ability to block light. The mean free path is a concept often presented when discussing the Kinetic Molecular Theory in a first year chemistry course. Within the dielectric formalism, the IMFP is defined by an integral.The mean free path of particles of a gas equation is mentioned-below: ╬╗ = k * T / (ΓêÜ2 * ╧Ç * d┬ * p) Where, ╬╗ is the mean free path. In 2015, an analytical formula for IMFPs was derived from this . Learn how to calculate it using the exponential factor and the constant μ, and see examples of mean . To see this, note that the probability that the a particle is . The exact values, however, are a little trickier to calculate. Because most real .380649 x 10-23 J/K) T = temperature of the gas; d = diameter of the particle; p = pressure of the gas; To do appropriate computations, the mean free path formula requires atomic diameter .Schlagwörter:Mean Free PathMean Free Time Between Collisions
Mean Free Path Calculator
Momentum and energy are conserved in these collisions, so the ideal gas law remains valid. From the equation for the probability of the first collision in dx ( P (x)dx = Σ t dx .The following is the mean free path of particles in a gas equation λ = k * T / (√2 * π * d² * p) Where, λ = mean free path; k = Boltzmann constant (k = 1.The mean free path can be defined as the product of the average speed and the mean free time, where the mean free time is the average time between collisions.A new equation for the mean free path of air is also proposed as a function of temperature and pressure, namely λ (T, P) = 0.Mean free path is defined as a gas molecule’s average travel length between collisions. The problem is that the integration is rather complicated. mfp 일명 평균자유행로 라 일컬어지는 이 말은 가스 원자들이 다른 가스 원자를 만나 충돌할 수 있는 평균 거리를 나타낸것을 의미한다. The evolution of the probability density distribution of free paths for observation times t obs from 1 to 30 ns at T = 3000 K and P = 1 atm is shown in Figure S5.As the formula for the mean free path is already known, that is, λ = N/V is the number density that can be equated to P/KT by ideal gas law, Therefore, λ = λ = λ = . d is the diameter of the particle.The inelastic mean free path (IMFP) is an index of how far an electron on average travels through a solid before losing energy. The origin of this equation can be understood as follows. This is defined as the product of the speed of a particle .Schlagwörter:Mean Free PathMean Free Time Between Collisions
Mean Free Path
To do so, more careful definitions are needed, and one should carefully calculate averages of functions of particle velocities. We want now to discuss what happens when things are near, but not exactly in, equilibrium. See the formula, derivation and examples of mean free path in physics.Learn what is mean free path, the average path length between collisions of gas molecules, and how to derive its formula.The electron inelastic mean free path (IMFP) is the average distance between successive inelastic collisions of an electron moving in a medium with a given energy.
- Mechernich Tourismus – Wandern & Walken
- Median Klinik In Bad Salzuflen
- Mediathek Filme Kostenlos Anschauen Neu
- Medal Of Honor Pdf , Medal of Honor™: Above and Beyond
- Mechatronik Ausbildung Gehalt : Mechatroniker : Einstieg, Aufstieg, Einkommen
- Mdt To Est Time Converter , MDT to ET Converter
- Mb Ml 320 Gebraucht – Mercedes-Benz B-Klasse Gebrauchtwagen
- Mechatronik Hochschule Würzburg
- Mediacreationtool | Create installation media for Windows
- Medela Schlafbustier _ Medela: Infos zur Marke & Produkte kaufen
- Mbox Mit Outlook Öffnen _ MBOX in Outlook Importieren
- Media Player Aktivieren : Windows Media Player: 64 Bit aktivieren