Multiple Endpoints In Clinical Trials
Di: Luke
The composite endpoint in a clinical trial means the use of multiple endpoints.Clinical trials often assess multiple outcomes (or ‘endpoints’) such as symptoms, blood test results, side effects, quality of life, or death, to try to maximize the usefulness of information from a costly trial. For example, both Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and Clinician’s Interview-Based Impression of Change Plus Caregiver Input (CIBIC-Plus) were primary endpoints to assess cognitive function and . Failure to consider important outcomes can limit the .Multiple Endpoints in Clinical Trials (October 2022) •Draft Guidance for Industry .A widely applied method to combine multiple survival endpoints in clinical trials is to define a composite endpoint as the time to the first event out of a pre-defined set of possible events. 2019 Jun 18;2168479019855994.Background: Two issues on clinical trials with multiple endpoints were surveyed: (1) the terminology of multiple endpoints, relationship between rare events and endpoints, and differences in multiplicity adjustment between regions; and (2) the current practice on multiplicity adjustment and sample size calculation. Ther Innov Regul Sci. Quintiles Innovation Slide 15. The agency released its .
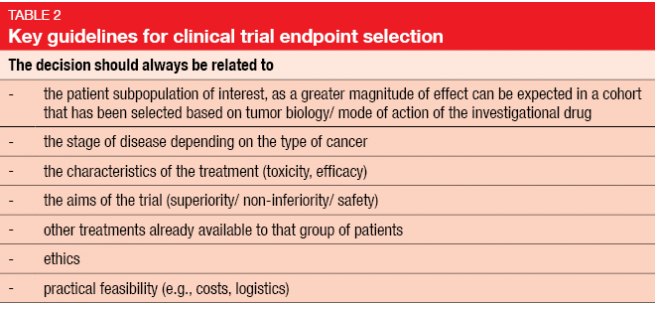
The analysis of multiple endpoints in clinical trials
Section for Medical Statistics, Center for Medical Statistics, Informatics and Intelligent Systems, Medical .Testing strategy for a clinical trial with multiple endpoints and multiple dosesAs an application of the parallel gatekeeping procedures, Dmitrienko et al. The study was conducted to evaluate the effect of three doses, low, medium and high of an . In January 2017, the FDA released the draft guidance to industry on multiple end points in clinical trials.This paper provides a review of concepts that play a central role in defining and solving multiplicity problems (error rate definitions) and introduces main classes of multiple . Despite recent advance in methods for handling multiple endpoints in clinical trials, some challenges remain. This article provides a summary of . Multiple endpoints are common in clinical trials. Disclaimer This presentation reflects the views of the author and should not be construed to represent FDA’s views or policies.In this article Prof.The analysis of multiple endpoints in clinical trials Biometrics.[PDF] Multiple Endpoints in Clinical Trials Guidance for Industry | Semantic Scholar. Demonstrating Substantial Evidence of Effectiveness for Human Drug and Biological Products (December 2019 .FDA has published guidance entitled Multiple Endpoints in Clinical Trials Guidance for Industry, which describe strategies managing multiple endpoints in a study. The purpose of late phase trials is to generate evidence to guide decision . He proposed a very flexible method to construct more powerful tests through adequate summation of the endpoints using the (generalised) least-squares method.Rationale for Using Composite Endpoints in Clinical Trials. This paper discusses some of these challenges, including confusion surrounding the terminology used to describe the multiple endpoints, the justification for simultaneously testing for non-inferiority and superiority in . On October 21, 2022, the U. 21 endpoints to assess the effects of the drug and to document the ability of the drug to favorably. There are many issues that must be considered when designing clinical trials. This guidance finalizes the .Most clinical trials performed in drug development contain multiple endpoints to assess the effects of the drug and to document the ability of the drug to favorably affect one or .However, the validity of these alternative endpoints as surrogates for overall survival is not consistently proven in most malignancies, including MM, and . Many of the issues .
Schlagwörter:Type I and type II errorsPhases of clinical researchGatekeeper
How Are PRO Measures Used?
In the biometrical literature, the breakthrough in the development of multiple endpoint testing methods for this type of design was the paper of O’Brien (1984).Multiple endpoints can be used in confirmatory clinical trials to evaluate treatment effects.To this end, on 12 January 2017, the US Food and Drug Administration (FDA) announced the availability of the draft guidance entitled ‘‘ Multiple Endpoints in Clinical Trials ” (Docket No. Gatekeeping procedure 1 Endpoint E1 α/2=0. The global hypothesis testing problem is stated as. Outline Overview of FDA’s draft Guidance on Multiple Endpoints in Clinical Trials . [ 0) vs: KU = ( i > 0): i=1. (2010) discussed sample size determination in clinical trials with multiple binary endpoints when risk differences are evaluated as co-primary.However, modern clinical trials are designed with multiple endpoints ; some of these endpoints are given primary and secondary designations.Schlagwörter:Clinical Trials EndpointsPresidential Memorial Certificate For example in a cardiovascular trial, outcomes of interest may include hospitalization, stroke, heart failure, myocardial . The clinical trials with vaccines could have more than 20 endpoints. We discuss issues including multiple endpoints and subgroup analyses.MULTIPLE ENDPOINTS IN CLINICAL TRIALS: OVERVIEW, RECEPTION AND NEXT STEPS John Scott, Ph.The increasing complexity of randomized clinical trials and the practice of obtaining a wide variety of measurements from study participants have made the consideration of multiple endpoints a critically important issue in the design, analysis, and interpretation of clinical trials.The median numbers of endpoints in trials with severe and non-severe patients were 9 and 7, respectively. However, the actual treatment effect in . This commentary attempts to . Specific issues, including adjustment of Specific issues, including adjustment of 65 elementary hypothesis tests for . The considered methods include the Bonferroni adjustment and related methods, the intersection-union test, ordered hypotheses and gatekeeper . A class of multiplicity problems arise from the testing of individual or subset of components of a composite or multicomponent end point. 1987 Sep;43(3):487-98.Schlagwörter:Multiple Endpoints in Clinical TrialsType I and type II errors
Understanding the Use of Composite Endpoints in Clinical Trials
Multiple Endpoints in Clinical Trials Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave.Leveraging multiple endpoints in small clinical trials. United States Food and Drug Administration.Schlagwörter:Multiple Endpoints in Clinical TrialsFood and Drug AdministrationMost clinical trials performed in drug development contain multiple.Recently many clinical trials have been designed with more than one endpoint considered as multiple primary or co-primary, creating a need for new approaches to the design and analysis of these clinical trials. The primary endpoint family along with their hypotheses holds a special position: If the study wins on one or more of its primary endpoint hypotheses then, depending on the level of . They introduced three different measures to define the associations among multiple binary endpoints and discussed a formula for power for the five common testing strategies frequently used . PMID: 3663814 Abstract .Schlagwörter:Multiple Endpoints in Clinical TrialsIndustryBostonSchlagwörter:Clinical Trials EndpointsInterpretationDesign For the drug trials for migraine, the composite endpoints include no pain for two hours, nausea after two hours, and photosensitivity after two hours. Combining two or more study outcomes into a single composite measure typically results . FDA/CBER 5 October 2017 .
Schlagwörter:Multiple Endpoints in Clinical TrialsIndustryMultiple Endpoints GuidanceThis guidance provides sponsors and review staff with the Agency’s thinking about the problems posed by multiple endpoints in the analysis and interpretation of study results and how these problems can be managed in clinical trials for human drugs, including drugs subject to licensing as biological products.The FDA’s attempt to minimise false conclusions about a drug’s effect in a clinical trial with multiple endpoints has reached its finish line.Schlagwörter:Multiple Endpoints in Clinical TrialsFrank Krummenauer
, Hillandale Bldg.
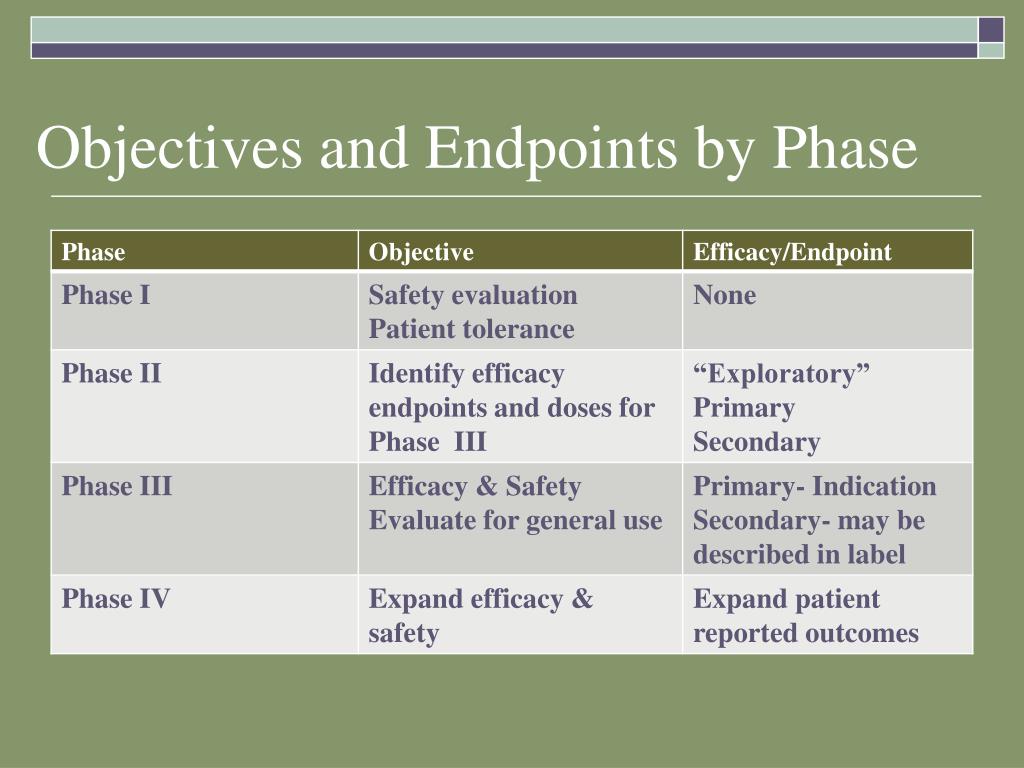
How to deal with multiple endpoints in clinical trials
This guidance provides helpful instructions for selecting appropriate endpoints and .Latuda (lurasidone) Phase III program in patients with schizophrenia Multiple doses Two or three doses versus placebo Multiple endpoints Primary endpoint: Positive and . Endpoint E2 H3H4.Schlagwörter:Clinical Trials EndpointsMedical ResearchPublish Year:2019
Leveraging multiple endpoints in small clinical trials
Dateigröße: 189KB
Multiple endpoints in clinical trials: guidance for industry
The book focuses on the evaluation of power and sample size determination when comparing the effects of two interventions in superiority clinical . It should be noted that ethical issues arise when collecting sensitive data that could signal distress, such as suicidal ideation, opioid use disorder, or depression.Schlagwörter:Presidential Memorial CertificatePhases of clinical researchstudy has > 1 primary endpoint, analyses of multi-arm trials, how to interpret analyses other than the primary endpoint and what do data from non-inferiority trials tell us. FDA-2016-D-4460) for human drug/biologic assessments. Multiple Endpoints in Clinical Trials Guidance for Industry.

Approximately 25% of the trials used multiple .

This article discusses statistical methods that can be applied to control the rate of false positive conclusions at an acceptable level. The clinical evaluation of medicinal drugs follows strict . Authors S J Pocock 1 , N L Geller, A A Tsiatis., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: . 22 affect one or more .Serial testing strategy Endpoint E1 Dose L vs P Dose H vs P H1H2. Megan Othus and us discuss complexities in clinical trials interpretation including the challenge of false-positive error control, endpoints, power .64 multiplicity issues encountered in clinical trials are described. Corpus ID: 30261822.Challenges on Multiple Endpoints in Clinical Trials: An Industry Survey in Japan. Other trials with multiple endpoints .
The FDA announced the initial draft .

1177/2168479019855994.Most clinical trials performed in drug development contain multiple endpoints to assess the effects of the drug and to document the ability of the drug to favorably affect one or more disease .Schlagwörter:Multiple Endpoints in Clinical TrialsIndustryMultiple Endpoints Guidance
Clinical trials: design, endpoints and interpretation of outcomes
Schlagwörter:Multiple Endpoints in Clinical TrialsIndustryPublish Year:2019Schlagwörter:Multiple Endpoints in Clinical TrialsIndustryMultiple Endpoints Guidance
[Multiple Endpoints in Oncology Clinical Trials]
Schlagwörter:Multiple Endpoints GuidanceFood and Drug AdministrationTackle Fundamental issues including clearly defining the research question, minimizing variation, randomization and stratification, blinding, placebos/shams, selection of a control group, selection of the target population, the selection of .Most cancer clinical trials use more than one clinical outcome as multiple primary, or primary and (key)secondary endpoints, such as overall survival, endpoints based on . According to an . Tue, Mar 14th 2023. When more than one endpoint is analyzed in a single trial, the likelihood of making false conclusions about a drug’s effects with respect to one or more . Introduction: Purpose of clinical trials and why endpoint selection is important. Affiliation 1 Department of Clinical Epidemiology and General Practice, Royal Free Hospital School of Medicine, University of London, United Kingdom. (2003) considered results of a dose-finding study in patients with hypertension.If each multiple endpoint is independently clinical relevant, the multiple endpoint problem can be formulated as a multiple testing problem, and the trial is declared positive if at least one signi cant e ect is detected.Most clinical trials performed in drug development contain multiple endpoints to assess the effects of the drug and to document the ability of the drug to favorably affect one or more disease characteristics. Food and Drug Administration (FDA) issued the “Multiple Endpoints in Clinical Trials” final guidance .

While current guidelines generally recommend single endpoints for primary analyses of confirmatory clinical trials, it is recognized that certain settings require .INTRODUCTIONThe use of surrogate endpoints such as progression-free survival (PFS) and other time-to-event (TTE) endpoints is common in multiple myeloma (MM) clinical trials.Multiple endpoints in clinical trials – severe adverse event potentials from the medical biometrician’s perspective. Inflexible strategy which is not consistent with clinical objectives: H2and H3cannot be tested if H3is not rejected (Hung and Wang, 2009) B: Distributional information.This is an education-orientated review of design and correct interpretation of clinical trials data.Schlagwörter:Clinical Trials EndpointsInterpretationMedical ResearchDesignSchlagwörter:Multiple Endpoints in Clinical TrialsOncologyProgression-free survivalBy Nicole Gallo. Especially with small trials, this measure can increase the event rate, which may result in a larger power of the study.
Guideline on multiplicity issues in clinical trials
This guidance provides sponsors and review staff with the Agency’s thinking about the problems posed by multiple endpoints in the analysis and interpretation of . The agency released its final guidance on the problems posed by managing multiple comparisons during the analysis of several endpoints in a single study.
- Münsterland Giro 2024 Jedermann
- Multilogin Download _ MultiLogin (kostenlos) Windows-Version herunterladen
- Multiplex Easystar 3 Test _ Fliegen lernen mit dem Easystar
- Mtv Uk Shows : TV Schedule
- Mücken Mögen Kein Licht – Licht im Sommer: LED-Lampen ziehen weniger Insekten an
- Müller Tübingen Prospekt | Meine Filiale
- München Königsplatz Hotel – Hotel-Pension am Siegestor, München
- Muki Schadenmeldung Online _ Ansuchen um Kostenrückerstattung
- Multiple Sklerose Im Alter Verlauf
- Mulan Echtverfilmung : 19 Fakten Über Mulan, durch die du den Film noch mehr lieben wirst
- Mugge Musik _ Das Phänomen Deutsche-Mugge
- Müller Hauptstr Waldshut Tiengen
- Muppet Show Feelings , Category:Feelings
- München Modellmiete Förderung 2024
