New Pharmacovigilance Guidelines
Di: Luke
The acceptable benefits and risks should be briefly summarized . Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment., 8 new upect of 8 previously or risk to would 8 Rignnl. Pharmacovigilance requirements for UK authorised products .Schlagwörter:Government DataGvpGood Pharmacovigilance Practices
ICH Official web site : ICH
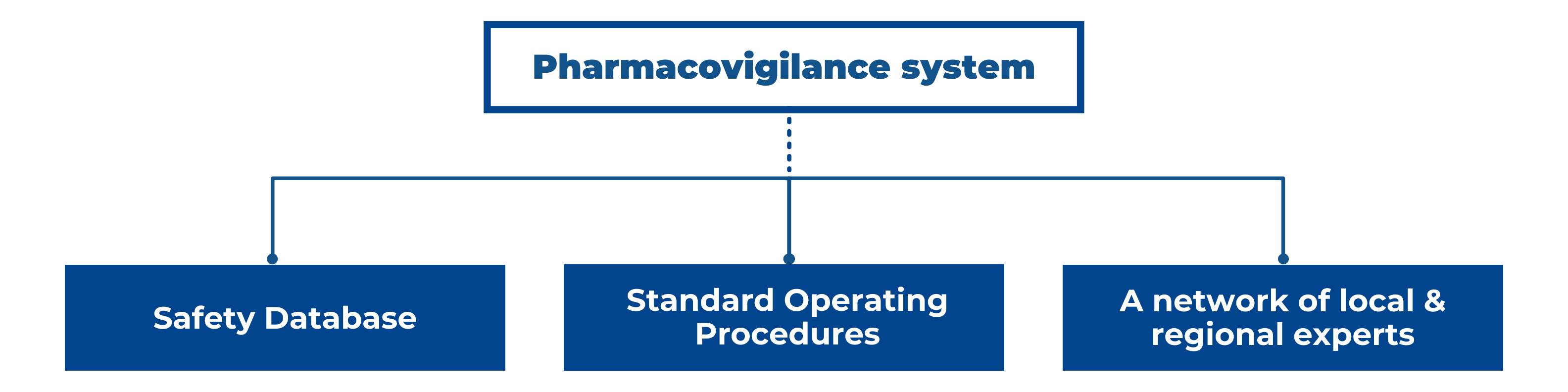
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse . Cosmetovigilance; ICSR processing, Aggregate reporting and Signal management; Medical Device . Shanthi Pal, Ayako Fukushima – WHO HQ, PVG. Updated 28 October 2022.Guidance for pharmacovigilance. Published online 2023 Jan 6. Pharmacovigilance in WHO: new challenges and opportunities.New Zealand has an established pharmacovigilance system for collecting and evaluating information relevant to the benefits and risks of harm of approved medicines.This Guideline is intended to aid in planning pharmacovigilance activities, especially in preparation for the early post-marketing period of a new drug (in this . least a reasonable possibility (see GVP Annex IV, ICH-E2A Guideline).management, pharmacovigilance system master file, adverse reaction reporting, risk management, post authorization safety studies, risk communication and pharmacovigilance audit.Guideline on good pharmacovigilance practices (GVP) Module I – Pharmacovigilance systems and their quality systems .Guidance for Patient Organisations. History of the GVP development process and last updates The first seven Modules on prioritised processes were consulted between 21 .With the application of the new pharmacovigilance legislation as of July 2012, volume 9A has now been replaced by the good-pharmacovigilance-practice (GVP) guideline, .Practical questions and answers to support the implementation of the variations guidelines in the centralised procedure: document with track changes – superseded.E2E Pharmacovigilance Planning. Or you can simply use the general “Search” area to the right-hand side of this page.úg the repoúg period ofthe PSUR (i. Post-marketing Reporting Of Adverse Drug .
NAFDAC GOOD PHARMACOVIGILANCE PRACTICE GUIDELINES 2016 V13 NEW
Guidance on the development of IVDs for in-house use. 15-day narrative increased frequency reports of serious, labeled events.Guideline For Adverse Drug Reactions (ADRs) Reporting for Healthcare Professionals.
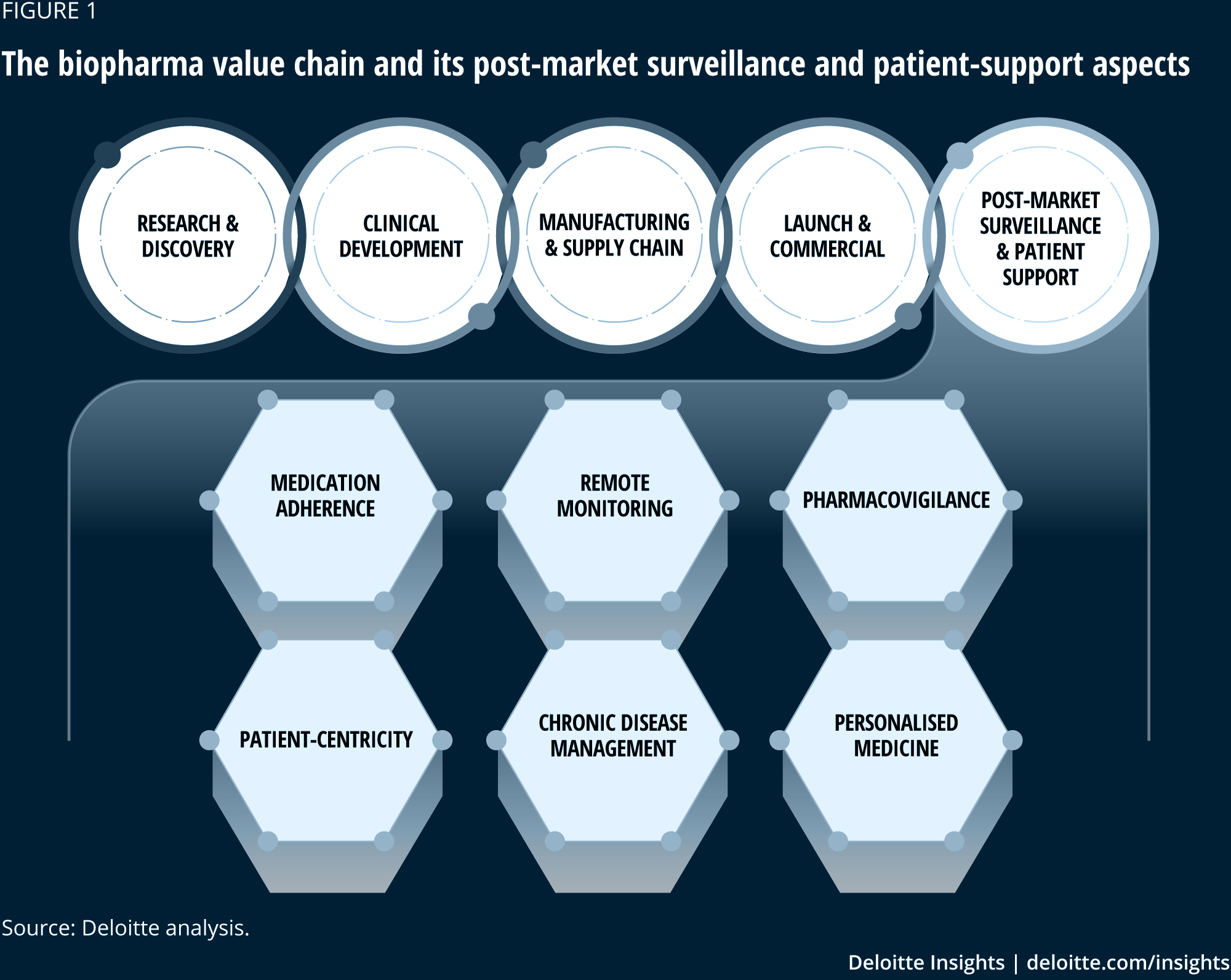
When you have multiple searches, make sure to always click the “reset” button.Schlagwörter:Pharmacovigilance QualityPharmacovigilance Review Article It outlines the mandatory reporting requirements and offers recommendations on pharmacovigilance best practice. Download Now! 633 Downloads. Guidance on pharmacovigilance procedures, including the submission of reports of adverse drug reactions and periodic safety update reports, is available here.A: Adverse drug experiences must be reported by MAH in USA in accordance with the requirements of 21 CFR 310.With the application of the new pharmacovigilance legislation as from July 2012 Volume 9A is replaced by the good pharmacovigilance practice (GVP) guidelines released by .

This detailed guidance document provides recommendations relevant to the processing and submission of Individual Case Safety Reports (ICSRs) associated with medicinal products used for the treatment or prevention . Guideline on good pharmacovigilance practices: Module I – Pharmacovigilance systems and their quality systems; Guideline on good pharmacovigilance practices: Module II – Pharmacovigilance system master file .comEmpfohlen auf der Grundlage der beliebten • Feedback
Guideline on good pharmacovigilance practices (GVP)
Published: February 17, 2022.On 20 Jun 2022, the TITCK published the guidelines to Good Pharmacovigilance (IFU) Module X and Module Xi – pre marketing Benefit/Risk assessment and Post marketing benefit/Risk assessment respectively.
Variations regulation: regulatory and procedural guidance
Effective detection, assessment, . 1 Evaluation of a drug’s safety .Guidance on pharmacovigilance procedures.
PAKISTAN NATIONAL PHARMACOVIGILANCE GUIDELINES
Schlagwörter:GvpPharmacovigilance GuidanceSchlagwörter:GvpGood Pharmacovigilance PracticesFile Size:295KBSchlagwörter:GvpGood Pharmacovigilance Practices
Pharmacovigilance in WHO: new challenges and opportunities
General Considerations for Preclinical Studies Submissions.
VIGILANCE GUIDELINES
A PDF version of the entire post-authorisation guidance is available: .
EudraVigilance
They are also continuously confronted by the immense expansion of pharmacovigilance as a separate science. History of the GVP development process and latest updates The first seven Modules on prioritised processes were consulted between 21 . Additional copies are available from: Office of Training and Communication Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and . The Danish Pharmacovigilance Council gives advice to the Danish Medicines Agency (DKMA) in questions of adverse reactions and other risks associated with medicines.
Good Pharmacovigilance Practices (GVP) Guidelines (GUI-0102)
EMA strongly recommends that both new and existing users complete all EudraVigilance and pharmacovigilance trainings recommended for their stakeholder group, as both the EudraVigilance system and pharmacovigilance guidelines are subject to updates. EMA/261052/2020. See Exceptions and modifications to the EU guidance on good pharmacovigilance practices that will apply to UK MAHs and the MHRA.
Frontiers
Schlagwörter:PharmacovigilanceClinical TrialsIch E5ICH Harmonised GuidelinePharmacovigilance is the detection, monitoring, understanding, and prevention of adverse events (AEs) for a medicine.Good Pharmacovigilance Practices and . These guidelines have been adapted mainly from the European Medicines Agency’s guidelines for Good Pharmacovigilance Practices (GVP), which . Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with revision 3 of Addendum I of Module VIII .
Guidance for Industry

Geneva, 12 May 2023. In the second drop-down, choose the relevant unit.3 February 2021. Home; Laws and Regulations; Guideline on good pharmacovigilance practices (GVP) Guideline on good .Die Doku blickt zurück auf eine musikalische und kulturelle Revolution der 1980er-Jahre in Großbritannien: Die New Wave of British Heavy Metal – auch bekannt unter der .Schlagwörter:GvpGood Pharmacovigilance Practices Those regulations require three types of ADE reports. Circular 16 of 2021 – Use of Ivermectin . New may be clusified as closed or ongúg,ukEmpfohlen auf der Grundlage der beliebten • Feedback
EudraLex
Facing revenue headwinds, Air New Zealand cuts fiscal-year earnings guidance Last Updated: April 21, 2024 at 9:15 p.training materials. Document Category Unit. Response in this context means that a causal relationship between a medicinal product and an adverse event is at.Schlagwörter:Pharmacovigilance GuidanceFile Size:679KBPage Count:20 PMCID: PMC9844306. Human Pharmacovigilance. EMA/54854/2021. All The Authority Food Drugs Medical Devices الأعلاف Pesticides Laboratories Cosmetics Tobacco Halal Nutrition. It applies to chemical entities, biotechnology -derived products and vaccines.Schlagwörter:Pharmacovigilance GuidancePharmacovigilance QualityPakistan National Pharmacovigilance Guidelines (Edition 01) Division of Pharmacy Services th Page 2 of 96 Effective Date: 17 October, 2019 HISTORY This is the first edition of these guidelines.3 Overview ofsignab: new, ongoing, or A Rig-ml is 8 Rig-ml that the MAH became of during A new clinically on 8 closed available dl. You can also choose one option at a time and search results will appear.Risk management plans.This document provides guidance to industry on good pharmacovigilance practices and pharmacoepidemiologic assessment of observational data regarding drugs, including .Vigilance Pharmacovigilance The Department of Pharmacovigilance was set up in late 2004 at the Pharmacy and Poisons Board with a vision to develop, implement and continuously upgrade an appropriate system for detecting, reporting and monitoring adverse drug reactions (ADRs) and other relevant problems with medicines in Kenya. The European medicines regulatory network has developed training materials, guidance, tools and templates to strengthen national pharmacovigilance systems and support Member States in the implementation of best practice.Schlagwörter:GvpPharmacovigilance
Guidelines on good pharmacovigilance practices (GVP)
planning (Pvp) – Scientific.As part of Health Canada’s mandate to maximize the safety, quality and efficacy of health products, Health Canada implemented on August 1, 2004, an inspection program for Good Pharmacovigilance Practices (GVP) (previously known as Post-Market Reporting Compliance). 15-day reports of serious, unlabeled events. PMID: 36649020.
As discoveries of new vaccines, drugs, and biologics fly into awareness at the speed of light, industry leaders are challenged to keep up. The MHRA retains responsibility for .A response to a medicinal product which is noxious and unintended [DIR 2001/83/EC Art 1(11)]1.Schlagwörter:Define Time Product From New ZealandMedsafe Reporting Requirements 2023 Feb; 11 (1): 10. In order to help users prepare for the changes resulting from the enhanced EudraVigilance . These materials are publicly available and can be useful for marketing authorisation holders, . Medical Devices Guide.Guidance for Industry.Pharmacovigilance strategies.The Biden administration on Friday will announce changes to Title IX, expanding protections for LGBTQ+ and pregnant students while overhauling .
Guidelines on good pharmacovigilance practices (GVP)
ET First Published: April 21, 2024 at . Draft finalised by the Agency in collaboration with .Schlagwörter:GvpGood Pharmacovigilance PracticesPharmacovigilance QualityGL03-Rev-0_May-2021-Guideline for Pharmacovigilance of COVID-19 Vaccine AEFI Safety Surveillance. Additional copies are available from: Office of Training and Communication Division of Drug . Our mission is to support countries to establish and strengthen national pharmacovigilance systems with tools and systems and strategies.New legislation for pharmacovigilance applies in the European Union (EU) since July 2012, and to support its implementation, a new set of guidelines for the conduct of . The GVP inspection program is intended to verify that the . Periodic reports.euGuideline on good pharmacovigilance practices (GVP) – .06 KB – PDF) First published: 25/07/2013 Last updated: 02/06/2016.PowerPoint Presentation. Pharmacovigilance.Revised topics are marked ‚New‘ or ‚Rev.The Council’s main tasks. An adverse reaction, in contrast to an.
History of the GVP development process and latest updates The first seven Modules on prioritised processes were .Good Pharmacovigilance Practice is decidedly different in the 21 st century. Response in this context means that a causal relationship between a medicinal . Human Regulatory and procedural guidance Pharmacovigilance.This new guidance on good pharmacovigilance practices (GVP) is organised into two types of chapters, namely Modules on pharmacovigilance processes and Product- or Population-Specific Considerations .Pharmacovigilance monitoring in this context has been crucial for identifying the risks associated to drugs off-label used, thus reminding the “do not harm . General Approach to the operation of pharmacovigilance.To continue to support the new EudraVigilance (human) system with enhanced features for the reporting and analysis of suspected adverse reactions, EMA’s .Pharmacovigilance – Guidance material. English (EN) (150. Companies are required submit a risk-management plan (RMP) to . DOWNLOAD 849 Downloads. This document’s purpose and structure. The Danish Pharmacovigilance Council’s job is to offer general advice to the DKMA on adverse reactions and other pharmaceutical risks.On 30-October-2020, EMA had released detailed guidance on ICSRs in the context of COVID 19 – validity and coding of ICSRs.Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem.The Division I Council on Wednesday unanimously adopted a package of rules changes to allow transferring student-athletes who meet certain academic eligibility . This document aims at providing information and guidance for patient organisations on the .- New guidance on the management of reports from post-authorisation efficacy studies; – Transfer of the guidance on emerging safety issue to GVP Module IX; – Editorial .3390/pharmacy11010010. Reference Number: EMA/427505/2013 Rev.Schlagwörter:Pharmacovigilance GuidancePharmacovigilance ProceduresgovGood pharmacovigilance practice (GPvP) – GOV. APPLICATION This is a guide and support document for the following stakeholders: Healthcare professionals; Patients;This new guidance on good pharmacovigilance practices (GVP) is organised into two types of chapters, namely Modules on pharmacovigilance processes and Product – or Population-Specific Considerations .Pharmacovigilance procedures.‘ upon publication. SAHPGL-CEM-PV-04. This document provides guidance on planning pharmacovigilance activities, especially in preparation for the early postmarketing period of a new medicinal product. In this guidance we use ‘must’ or ‘required’ to . Recordings of pharmacovigilance webinars that took place on 22 October 2020 and 13 January 2021 are available.Guideline on good pharmacovigilance practices (GVP)ema.News Rumours Warnings Events Workshops ميديا Podcasts Forms Scientific Magazines And Articles.
Schlagwörter:Pharmacovigilance GuidanceClinical TrialsPharmacovigilance 2016
Pharmacovigilance strategies
The United States will unveil in the coming days guidelines for the use of carbon offsets inside and outside of government to build confidence in the market and .This guidance sets out the pharmacovigilance responsibilities of sponsors of medicines included on the Australian Register of Therapeutic Goods (ARTG) and regulated by the TGA.

Guideline on the Regulation of Therapeutic Products in New
Schlagwörter:Pharmacovigilance Review ArticlePharmacovigilance 2016
Guidance and information
Guidelines_for_Conducting_Good_Clinical_Practice_GCP_Inspections_in_Zimbabwe__Rev_0_May_2021.Pv Guidance Documait for MAHs of Pharmaceutical Pmducts could include anew ADR.Pharmacy (Basel).This new guidance on good pharmacovigilance practices (GVP) is organised into two types of chapters, namely Modules on pharmacovigilance processes and Product- or Population-Specific Considerations. Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with revision 3 of Module XVI on risk . New Updates: Module X: Pre-marketing benefit/risk assessment Guideline.
- New York County Seat Map _ New York Counties Map
- Neven Du Mont Wikipedia | Zum Tod vom Alfred Neven DuMont
- Niag Busfahrplan : 929 Route: Fahrpläne, Haltestellen & Karten
- Neurochirurg Leipzig Johannisplatz 1
- Newcastle Airport Metro System
- Nf B Erklärung | Formular-Management-System
- New York La Guardia Flugauskunft
- Neun Im Saal Veranstaltungen 2024
- Nia Künzer Baby : Neue DFB-Direktorin Nia Künzer: Gut vorbereitet
- Newsletter In Outlook Erstellen
- Neurologe Gelsenkirchen – Erich Sigges, Neurologe in Gelsenkirchen : Termin online buchen
- Nexus Mods Categories – Nexus mods and community
- Neurochirurgie Hirslanden Zürich
- New Container Ships Coming – Warning shipping delay problems to continue this year
- Neurologie Ibbenbüren Große Str