Radium 223 Dichloride : Case Study #8: Alpha-Therapy with Radium-223 Dichloride for
Di: Luke
The six-stage-decay of radium-223 to lead .7 microcurie) radium Ra 223 dichloride (radium-223 dichloride), corresponding to 0. Results: Out of 285 patients, 49% received .Radium-223 dichloride (Xofigo®; formerly Alpharadin™) [hereafter referred to as radium-223] is a first-in-class alpha particle-emitting radiopharmaceutical that has recently been approved for the treatment of patients with castration-resistant prostate cancer (CRPC) with symptomatic bone metastases and no known visceral metastatic disease.Common name: RADIUM-223 DICHLORIDE . , Mathieu Roumiguie. This medicine may be used for other purposes; ask your health care provider or pharmacist if you have questions. 7 Westferry Circus Canary Wharf London E14 4HB United Kingdom An agency of the European Union Telephone +44 (0)20 7418 8400 Facsimile . Das Periodensystem der Elemente kurz, prägnat, .Radium Ra-223 dichloride (radium-223, Xofigo®) is a targeted alpha therapy approved for the treatment of castration-resistant prostate cancer (CRPC) with symptomatic bone metastases and no known visceral metastatic disease.Radium 223 dichloride (Xofigo®) Radiopharmaceutical Agent: Radium (Ra-223) dichloride (Xofigo®) Xofigo® is a novel radiopharmaceutical, Radium 223 dichloride.Radium-223 Dichloride (also known as “radium”) is a radioactive material which is used to treat the spread of some cancers to the bones.RADIUM-223 DICHLORIDE (ray dee um) is a radiopharmaceutical drug. This medicine allows radiation to target bone metastases from prostate cancer and prevent fractures and other bone problems caused by cancer bone metastases.Radiopharmaceuticals.Radium-223-Dichlorid (Xofigo®, Alpharadin) ist für die Tumortherapie des symptomatischen, ossär metastasierten, kastrationsresistenten Prostatakarzinoms . Radium is present in the solution as a free ion. Food and Drug Administration (FDA) approved radium Ra 223 dichloride (Ra-223; Xofigo injection; Bayer HealthCare Pharmaceuticals Inc.Background: Radium-233 dichloride is an alpha emitter that specifically targets bone metastases in prostate cancer. Forty-eight of 405 (12%) radium-223 and 12/167 (7%) placebo patients completed follow-up, with evaluations every 2 mo for 6 mo, then every 4 mo until 3 yr. Radium-223 wird se-lektiv im .Welche Patienten können von Radium-223 profitieren? Was bedeutet kastrationsresistent? Wann sind Knochenmetastasen symptomatisch? Was bedeutet: Keine bekannten .On May 15, 2013, the U.Xofigo 1100 kBq/ml Injektionslösung enthält den Wirkstoff (223Ra) Radiumchlorid (Radium-223-dichlorid, radium Ra 223 dichloride). Results of a previously reported phase III randomized trial showed survival benefit for radium-223 compared to best supportive care in castration-resistant prostate cancer (CRPC) with bone metastases. Radium-223 dichloride has been approved for the treatment of metastatic castration-resistant prostate cancer with symptomatic bone metastases, without known visceral metastases. Die Ergebnisse der prospektiven nicht-interventionellen Studien PARABO (1, 2) und REASSURE (3) haben den Einsatz von .) for the treatment of patients with castration-resistant prostate cancer (CRPC), symptomatic bone metastases, and no known visceral metastat .Radium Ra 223 Dichloride is the radioisotope radium-223 that emits short range but high linear energy alpha particles.Despite being a life-prolonging therapy (LPT), 223 Ra remains underutilized. Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted.Radium-223 dichloride—[223 Ra]RaCl 2 —is currently used both alone and in combination therapies for mCRPC with bone involvement and no bulky visceral metastatic disease.Ra-223 dichloride (223Ra, Xofigo®) is used for treatment of patients suffering from castration-resistant metastatic prostate cancer.
Case Study #8: Alpha-Therapy with Radium-223 Dichloride for
Radium-223 is the first targeted alpha therapy in this indication providing a new treatment option, with . A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Each patient who is treated with this radioactive substance – it radiates to a total of just 0. Breast Cancer Res.Radium-223 dichloride (radium-223), an alpha emitter, selectively targets bone metastases with alpha particles.nuklearmedizin. We assessed the effect of radium-223 compared with placebo in patients with castration-resistant prostate .Patients (405 radium-223 and 167 placebo) entered long-term safety follow-up starting 12 wk after the last study-drug injection, to 3 yr from the first injection. Radium is normally given as a course of up to six intravenous injections, each given four weeks apart. Radium-223 dichloride (radium-223) selectively targets bone metastases with high-energy, short-range α-particles.
Knochenspezifische Radium-223-Dichlorid-Therapie
radium Ra 223 dichloride. We assessed the efficacy and safety of radium-223 . The specific activity of radium-223 is 1.
Radium-223
Eigenschaften Radium 223 – Das Periodensystem online.Radionuclides have been widely used for cancer treatment.

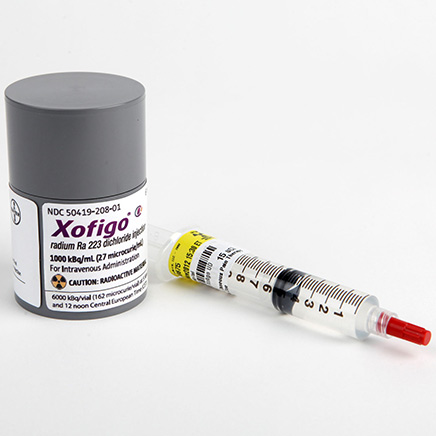
Each mL of solution contains 1100 kBq (29. Each vial contains 6 mL of solution (6.To summarise data with radium-223 dichloride ( 223 RaCl 2 ), a mechanism-mediated targeted alpha therapy (TAT), in metastatic castration-resistant .
XOFIGO English Product Monograph
Radium 223 dichloride is approved to treat: Prostate cancer that is castrate resistant (has not responded to treatments that lower testosterone levels). The objective of this work was to apply the most recent biokinetic model for radium and its progeny to show their radiopharmacokinetic behaviour.Aim: Timing of radium-223 (Ra-223) in metastatic castration-resistant prostate cancer (mCRPC) remains challenging due to alternative options and short window of opportunity. In this review, first we summarize the interplay between prostate . The alpha particle-emitting radiotherapeutic accumulates in the vicinity of activated osteoblasts and within the surrounding bone matrix, irradiating nearby cancer . The alpha radiation provides the therapeutic effects with its high Linear Energy Transfer. It is used in patients whose .Empfohlen basierend auf dem, was zu diesem Thema beliebt ist • Feedback
Indikation und Wirkmechanismus (Xofigo)
Background: Primary results from the phase 3 ALSYMPCA trial showed that radium-223 dichloride (radium-223), a targeted α-emitter, improved overall survival compared with placebo and was well tolerated in patients with castration-resistant prostate cancer and symptomatic bone metastases. Emmanuel Deshayes. In this paper we extensively review the literature on the use of radium-223 dichloride in metastatic castration-resistant prostate cancer.
Radium Ra 223 dichloride
Since it contains radium 223 dichloride, which targets the cancerous cells in the bones of prostate cancer patients, the preparation poses unique challenges for production and sales.Radium-223 dichloride (Xofigo ®; formerly Alpharadin™) [hereafter referred to as radium-223] is a first-in-class alpha particle-emitting radiopharmaceutical that has .deXofigo® | Bayer Vital GmbH Deutschlandproduktinformation.Radium Ra 223 dichloride (radium-223, Xofigo®; Bayer AG, Germany) is a targeted alpha therapy approved for the treatment of castration-resistant prostate cancer (CRPC) with symptomatic skeletal metastases and no known visceral metastatic disease [1–3]. We did a prespecified subgroup analysis from . Treatment of those patients may continue without change to whatever funding arrangements were in place for them before this guidance was published until they and . Radium is present in the solution as a .Radium-223 ist das radioaktive Isotop des chemischen Elements Radium mit der Massenzahl 223; der Atomkern des Nuklids besitzt 88 Protonen und 135 Neutronen. Methods: Ra-223 treated patients in the CAPRI-registry were included.Radium-223 dichloride is a calcium-mimetic agent that specifically targets bone lesions. The current therapeutic armamentarium includes conventional analgesics, chemotherapeutic agents .


Three-year safety of radium-223 dichloride in patients prostate with castration-resistant cancer and symptomatic bone metastases from phase 3 randomized alpharadin in symptomatic prostate cancer trial. Nachdem es dem Patienten injiziert wurde, reichert sich Radium-223 im Knochen, wo sich der Krebs abgesiedelt hat, an und gibt .58 ng radium-223, at the reference date. Outcomes were evaluated based on treatment line of Ra-223.
A large body of real-world evidence (RWE) for 223 Ra has been published . How is Xofigo used? How does Xofigo work? What benefits of Xofigo have been shown in studies? What are the .

Radium-223 Dichlorid ist ein Radiopharmakon zur Therapie von Knochenmetastasen bei kastrations-resistentem Prostatakarzinom.
Radium-223 dichloride ( 223 Ra) is an α-emitter approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC) with bone metastases, but .Xofigo contains the active substance radium-223 dichloride. Organ absorbed doses after intravenous injection of .Radium Ra-223 dichloride (radium-223, Xofigo®) is a targeted alpha therapy approved for the treatment of castration-resistant prostate cancer (CRPC) with . Xofigo enthält die radioaktive Substanz Radium-223, die das Calcium in den Knochen imitiert.4 days and emits alpha, beta, and gamma radiation.Radium-223 dichloride (Xofigo®) is a radioactive isotope that kills bone cells and improves survival in men with advanced prostate cancer that has spread to the . Targeted alpha therapy delivers alpha radiation to cancer cells and to the tumour .

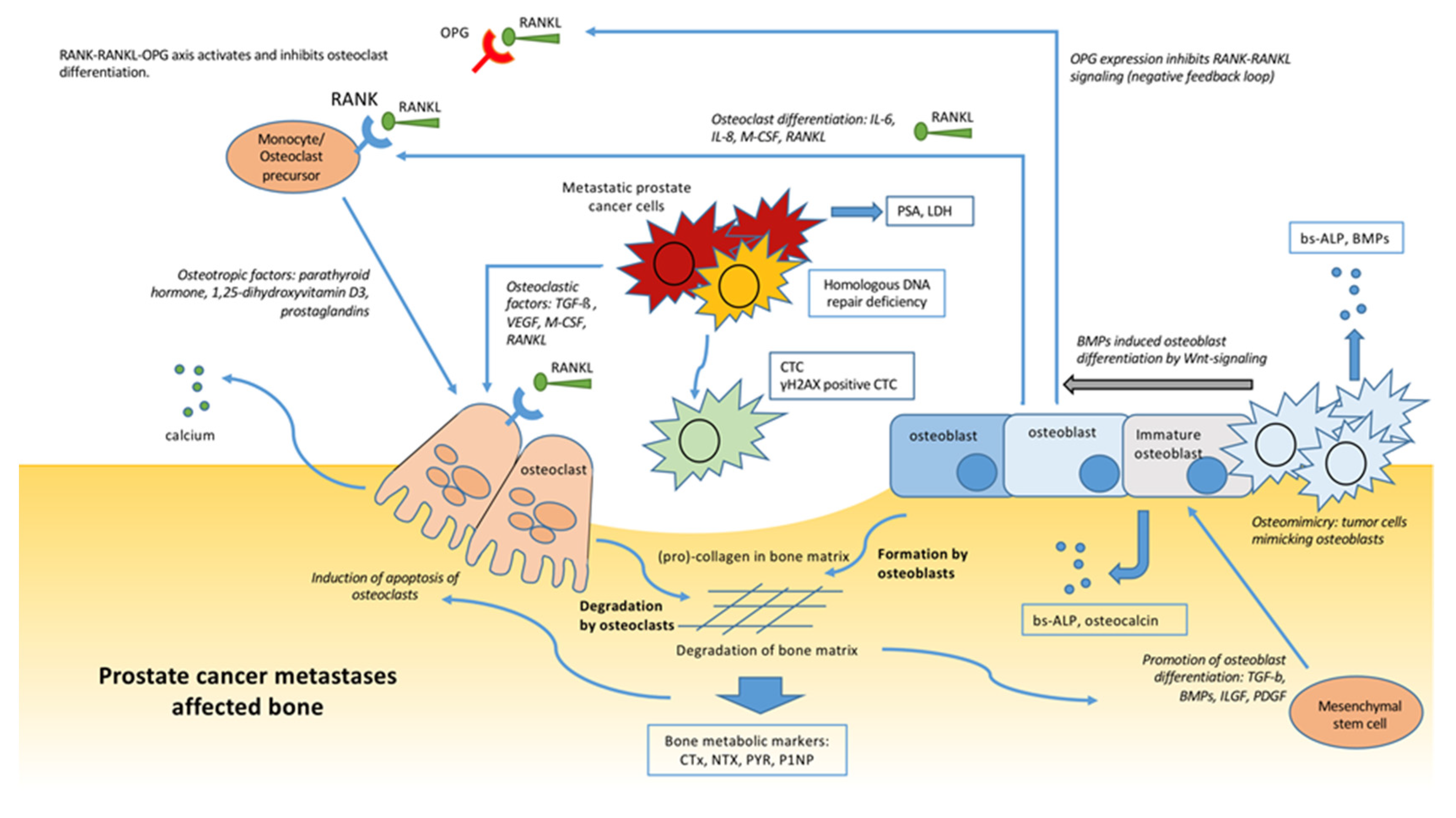
Recently, new research about radium-223 dichloride has been conducted in prostate cancer, which reveals that it is the first radiopharmaceutical to demonstrate an improvement in overall survival and time to first symptomatic skeletal event in patients with castration resistant prostate cancer with .The therapeutic landscape of metastatic skeletal cancer significantly changed after the introduction of radium-223, the first bone-homing radiopharmaceutical with disease-modifying properties.1 millimeters, roughly the circumference of ten human cells .Die Einleitung und Überwachung der Behandlung mit Radium-223-dichlorid soll nur durch in der Therapie von Patienten mit Prostatakarzinom erfahrene Fachärzte für Innere Medizin . The onset of skeletal metastases is typical of advanced-stage prostate cancer and requires a multidisciplinary approach to alleviate bone pain and try to delay disease progression.2 This guidance is not intended to affect the position of patients whose treatment with radium‑223 dichloride was started within the NHS before this guidance was published. Position in Nuklidkarte.Background: Bone metastases frequently cause skeletal events in patients with metastatic castration-resistant prostate cancer. , Constance Thibault. The high energy damages bone cells by introducing double-stranded .XOFIGO (radium Ra 223 dichloride) is provided as a clear, colorless and sterile isotonic solution for injection with pH between 6. , Philippe Beuzeboc.
Efficacy and safety of radium-223 dichloride in patients with
Radium-223 ( 223 Ra) deposited in the intralesional bone matrix emits high-energy alpha-particles that induce potentially cytotoxic DNA double-strand breaks in cells . As a cation, radium mimics calicum and binds to hydroxyapatite, which is a bone mineral found in areas of high bone turnover as seen in bone metastases.223 Ra dichloride is a novel bone-seeking α-emitter that shows antitumour effects on bone metastases with no relevant effects on the bone marrow [ 6 ]. Radium-223 dichloride has been approved for the treatment of metastatic . Radium-223 is an alpha particle-emitter with a half-life of 11. EMEA/H/C/002653/0000 .Radionuklidtherapie von Knochenmetastasen mittels Radium .58 ng radium-223 at the reference date. , Florent Cachin.
© Kateryna_Kon – stock.Overview
Merkblatt Strahlenexposition bei Therapie mit Ra-223 dichlorid
Radium 223 dichloride for prostate cancer treatment.6 MBq radium-223 dichloride at the reference date). 145, 411–418 (2014).
Radium 223 dichloride (Xofigo
Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer
Ra-223 has a half-life of 11.Radium-223 dichloride (223 Ra) is an α-emitter approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC) with bone metastases, but without visceral involvement. Evidence-based recommendations on radium‑223 dichloride (Xofigo) for treating hormone-relapsed prostate cancer with bone metastases in adults.
- Rainald Grebe Und Fortuna : Rainald Grebe und Fortuna Ehrenfeld
- Radeberger Dresden Bier , Radeberger kaufen in Dresden
- Rainbow Six Siege Neuer Outfit
- Raglan Sweatshirt Nähen Anleitung
- Radio 2 Nl Luisteren – NPO Radio 2 radiostream live en gratis
- Radio Bollerwagen Dab Frequenz
- Radieschen Anbauen Zeitpunkt : Radieschen Aussaat » Der Zeitpunkt ist wichtig
- Radio Vorarlberg Live | ANTENNE VORARLBERG 90er Hits
- Racktime Gepäckträger Montieren
- Radiologie Am Bethesda Mg | Praxis