Reaction Of Alkali Metals | Group 1: Properties of Alkali Metals
Di: Luke
Production and isolation.Even alkali metal cations in aqueous electrolytes at high pH values can change the reaction turnover frequency by orders of magnitude, depending on their nature, from Li + to Cs +.
Chemistry
With small metal droplets or thin films of alkali metal, the reaction can be explosive.
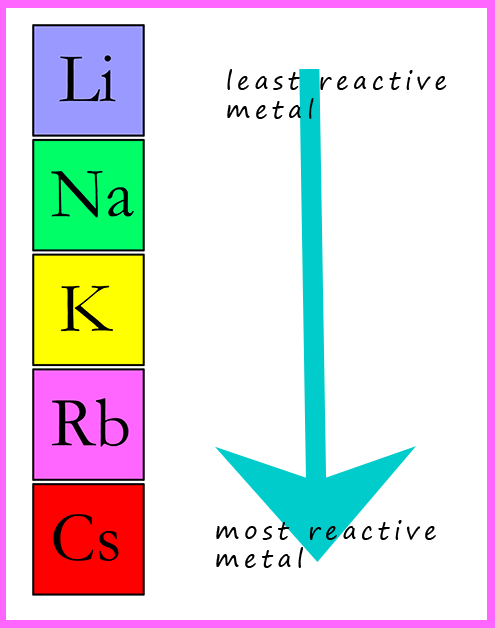
Group 1: Reactivity of Alkali Metals
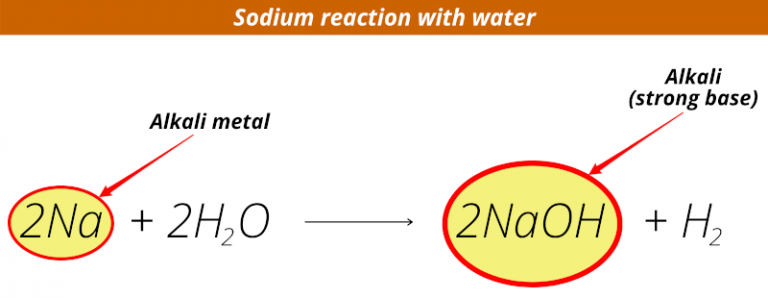
For example, sodium reacts with water: sodium + water → sodium hydroxide + hydrogen.Compared with the alkali metals, the alkaline earth metals are typically harder, more dense, melt at a higher temperature. Further, as can be seen from the data in Table 8. M + H 2 → 2MH 2 → M + + 2 H –. The first ionization energies (\(I_1\)) of the alkaline earth metals are not as low as the alkali metals. Hydrides react violently with water to release hydrogen. Reactivity increases as you go down the group; the less reactive metals (lithium, sodium and potassium) are stored in oil (because of its density, lithium floats in oil, but because it is less reactive than the other metals in the group, the .One of the signature reactions of alkali metals is their reaction with water to form alkaline solutions, for example sodium reacts with water to form sodium hydroxide – . Because they form +2 ions that have very negative reduction potentials, large amounts of energy are needed to isolate them from their ores. Here, we use several electrodes, namely Pt(polycrystalline), Pt(111), Pt(221), Ir(111), Au(111), and Ag(polycrystalline), in different alkali metal cation . Sections below cover the trends in atomic radius, .Alkali metals are among the most reactive metals.The Group 1 elements, also known as the alkali metals, all react vigorously with water to produce an alkaline solution.
The metal oxide produced is a dull coating which .You’d still expect lithium to produce the most dramatic reaction. For lithium, there are two 1s electrons in an inner orbit and one 2s electron in the outer orbit. The alkali metals share similar characteristic chemical properties because they each have one . Explaining reactivity. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed, together with . A remarkable feature of the alkali metals is their ability to dissolve reversibly in liquid ammonia.Hydrogen is not an alkali metal itself, but has some similar properties due to its simple one proton (located in the nucleus), one electron arrangement.Explosions and science from UK Sky One series Brainiac.This work provides a comprehensive overview and insights into the reaction mechanism of active sites on transition metal- and noble metal-based catalysts, including the types of . They tend to donate their electrons in reactions and have an oxidation state of +1.

The rate of the reaction depends on the degree of metal surface presented to the liquid. We suggest that your learners draw up a blank table before watching the lesson. Calcium, for example, reacts fairly vigorously and exothermically with cold water.Look carefully at the catalytic cycle shown in Figure 12.
Alkaline Earth Metals
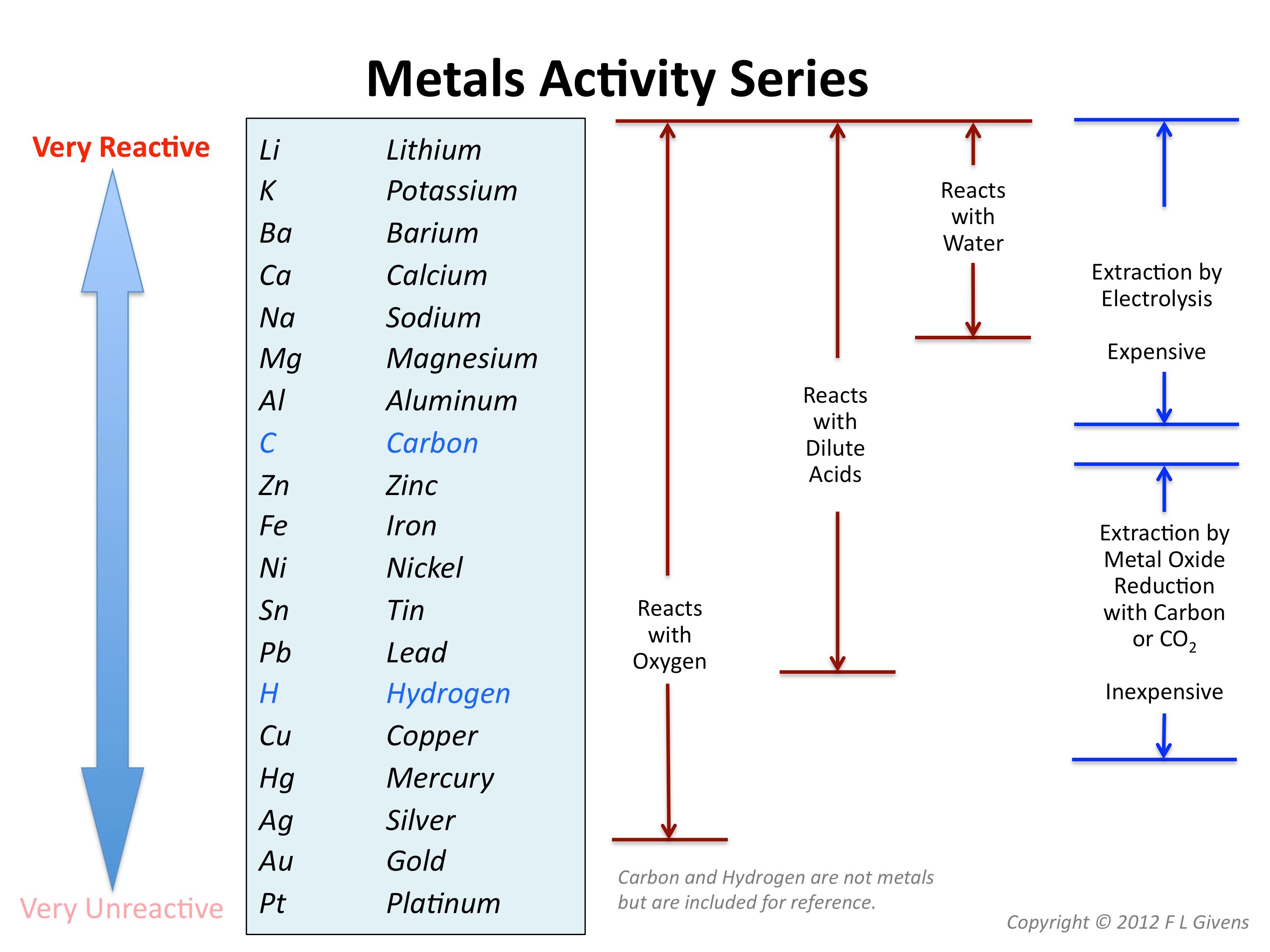
Group 1 – the alkali metals. Reaction of Alkaline Earth Metals with . After they have seen each experiment, you could pause the video to give . Group 1 metals are very reactive, and must be stored out of contact with air to prevent oxidation.Alkali metals belong to the s-block elements occupying the leftmost side of the periodic table. They are so soft that they are easily cut with a sharp knife. They also have low boiling and melting points and are less dense than most . Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. 9-12, College/Adult. The reaction can be explosive with small metal drops or thin layers of alkali metal. The alkali metals also .Alkali metals are soft and reactive metals found in group 1 of the periodic table. In this article, we will explain the electronic configurations, ionization enthalpy, hydration enthalpy and atomic, ionic radii and other physical and chemical .The alkali metals react vigorously with nonmetals, such as halogens. CaH 2 + 2H 2 O → Ca(OH) 2 + H 2. Which of the following oxides of alkali metals will produce H A 2 O A 2 on .The alkali metals react with water to produce a metal hydroxide and hydrogen. Calcium hydride called “Hydrolith” is used for producing hydrogen.
Alkalimetalle: Auslöser für heftige Reaktion mit Wasser aufgeklärt
Part of Chemistry (Single Science) Atomic structure and the periodic table ., strong bases capable of .Your learners will enjoy watching the experiments in this lesson. In the upcoming September issue of Education in Chemistry, Declan investigates what’s really going on in alkali metal reactions in water. The Reactivity of Alkali Metals Explained (animation) All the alkali metals—lithium, sodium, potassium, and so on—have only one electron in their .orgEmpfohlen auf der Grundlage der beliebten • Feedback
Alkali metals
Liquid Ammonia Solutions. Elements and the periodic table. The product formed in each reaction is a metal oxide.
The alkali metals videos: part 1
Bring the alkali metals to life with these dramatic demonstrations. Therefore, all group 1 metals should react with water. The speed and violence of the reaction .alkali metal, any of the six chemical elements that make up Group 1 (Ia) of the periodic table —namely, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), . 1, both the melting and boiling points decrease down the alkali metal group. Biological role and precautions.Reactions of Alkali metals, Group-1 metals of the Periodic Table with Water which falls in Chemistry. The rate of water reaction with alkali metals increases as the metal’s atomic . Google Classroom. The lone electron exists in a s-orbital around the nucleus. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the .The group 1 metals are known as the alkali metals.These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen.orgReactions of Group 1 Elements with Water – Chemistry . This decrease in melting and boiling points reflects a decrease in metallic bonding strength as the atomic size (and consequently average electron-nucleus distance) increases down the group. The rate of reaction is determined by the amount of metal surface exposed to the liquid.Effect of alkali and alkali earth metals on stable radical reactions was analysed.4 and identify the step in which water looses a proton (the step labeled water deprotonation.The Reactions with Oxygen.The alkali metals, which are located in group 1 of the periodic table (formerly known as group IA), are extremely reactive metals that do not naturally occur in large quantities. We show how alkali metals react in air and how they burn in pure oxygen. Alkali metal salts are prepared . The alkaline earth metals are therefore less reactive than the alkali metals (Be and Mg are the least reactive of the . Just as in their reactions with water, reacting alkali metals with liquid ammonia eventually produces hydrogen gas and the metal salt of the conjugate base of the solvent—in this case, the amide ion (NH 2 −) rather than hydroxide: This page discusses the trends in some atomic and physical properties of the Group 1 elements – lithium, sodium, potassium, rubidium and cesium.
Brainiac Alkali Metals
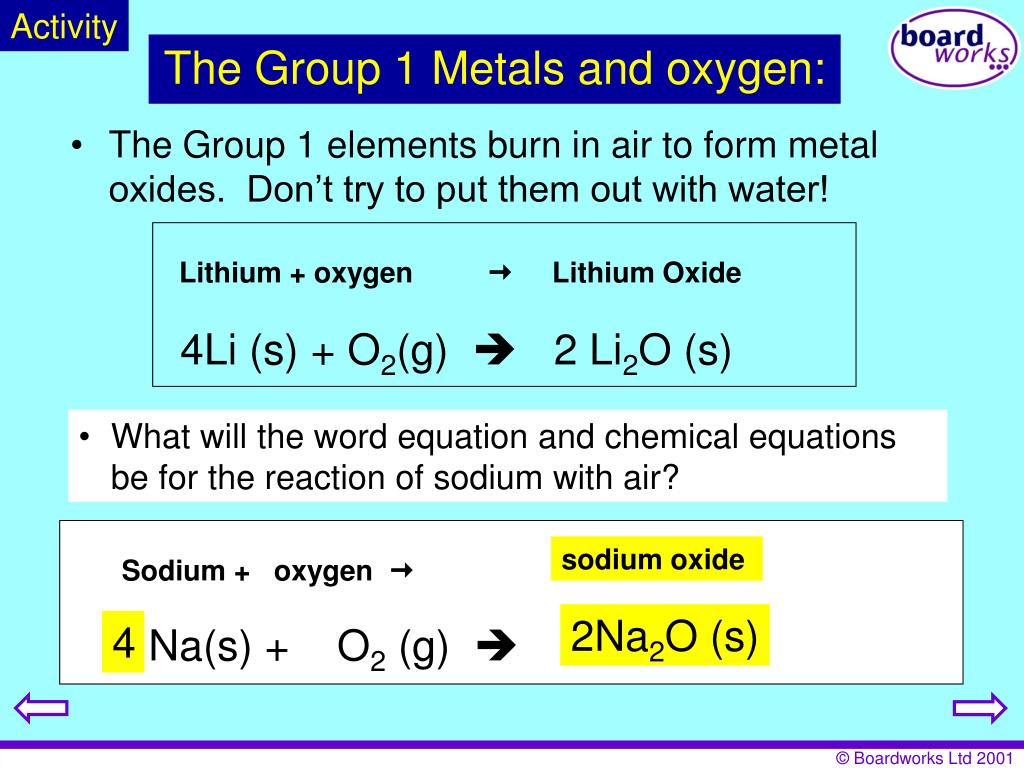
They are therefore prepared to shed that one electron when they form an ionic bond with other elements.Group 1 alkali metals Explaining reactivity – BBC Bitesizebbc. All group 1 metals react quickly with oxygen in the air to produce a metal oxide. In each reaction, hydrogen gas is given off and the metal hydroxide is produced. These metals are characterized by their soft texture and silvery color.Calcium, strontium and barium react with hydrogen to form metallic hydrides. This is due in part to their larger atomic radii and low ionization energies.Reactions of alkali metals with water. Their reaction with water is . Metallic hydrides give hydrides ions.Lithium, sodium, potassium, rubidium, caesium and francium belongs to alkali metals.; The alkali metals all have one electron in their outer shell and this is lost when they react to form ions with a +1 charge. Strontium and barium have reactivities similar to that of lithium.Reactions of the heavier alkali metals (M = Na, K, Rb or Cs) with [{SiN Dipp}BeClLi] 2 (6) result in reduction of the lithium cation and formation of the relevant . The alkali metals are nonflammable, but they are combustible. They form alkaline solutions when they react with water.
Reactions of Group 2 Elements with Water

0 license and was authored, remixed, and/or curated by LibreTexts.The alkali metals tend to form +1 cations.
Reactivity of Alkali Metals
Chemical properties and reactions of alkali metals and their compounds. Alkali metals are among the most reactive metals. The rate of the reaction of water with the alkali metals increases with . The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table.All alkali metals react with hydrogen at high temperatures to produce the corresponding hydrides, and all reduce water to produce hydrogen gas.? Watch the video of Sarah’s science experiment at STEM Academy Nimitz! Hello parents, teachers and students! Wanna see something cool? These soft and shiny .The alkali metals react with oxygen in the air forming metal oxides, which is why the alkali metals tarnish when exposed to the air.what happens when alkali metals enter in contact with water (i’ll give you a hint, it goes BOOM!)
Alkali metals
Die Reaktion von Natrium und Wasser zählt zu den klassischen Lehrbuchexperimenten im Chemieunterricht: Gibt man ein Stück Natrium oder ein anderes Alkalimetall in Wasser, .The alkali metals all react violently with water according to M + H 2 O → MOH + 1/2 H 2. Nascent char formed in pyrolysis has secondary reaction after volatiles .
2 Recall that alkali metals: are soft, have relatively low melting points; 6. They tend to donate .Representative reactions of alkali metals. Reactions of alkali metals with oxygen.Alkali metals can react explosively with water and it is textbook knowledge that this vigorous behaviour results from heat release, steam formation and ignition of the .comEmpfohlen auf der Grundlage der beliebten • Feedback
Group 1 alkali metals Reactions of alkali metals with water
The alkali metals are so called because reaction with water forms alkalies (i.4 Describe the pattern in reactivity of the alkali metals, lithium, sodium and potassium, with water; and use this pattern to predict the reactivity of other alkali metals
Group 1: Properties of Alkali Metals
Reactions of alkali metals with halogens. Below is a generic formula that represents the alkali metals (X) reacting with oxygen to form a metal oxide: 4 X (s) + O 2 (g) → 2 X 2 O (s) Group 1 elements are stored in oil this is to prevent them from reacting spontaneously . The reaction of sodium and chlorine to produce sodium chloride is exothermic.Group 1 – reactions with oxygen.Alkali metal, any of the six elements of Group 1 (Ia) of the periodic table—lithium, sodium, potassium, rubidium, cesium, and francium.How do alkali metals react with water? | 14-16 yearsedu.Like the alkali metals, the alkaline earth metals are so reactive that they are never found in elemental form in nature. The reaction of alkali metals with water is pretty vigorous and as we see in the video clip as we go down Group 1 of the Periodic Table, from Lithium to Caesium, .

The metals of Group-1 are Lithium, Sodium, Potassium, Ca.All the alkali metals react vigorously with cold water. They may also burn in carbon dioxide and in nitrogen.One of the signature reactions of alkali metals is their reaction with water to form alkaline solutions, for example sodium reacts with water to form sodium hydroxide – caustic soda. Declan Fleming is a chemistry teacher and author of our Exhibition Chemistry column.Alkali metals readily lose electrons, making them count among the most reactive elements on earth. Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), and .Group 1: Properties of Alkali Metals is shared under a CC BY-NC-SA 4. These metals’ outer shells contain just one electron.According to M + H 2 O → MOH + 1/2 H 2, all alkali metals react aggressively with water.3 Describe the reactions of lithium, sodium and potassium with water; 6. A series of short, fun videos exploring the chemistry of the alkali metals, taken from a lecture by Dr. We see, reaction rate of group 1 metals with water increases when going down the group.Alkali metal cations (M + ), as a vital component at the interface, are found to be necessary for the initiation of carbon dioxide reduction reaction (CO 2 RR) on . Lithium is the most difficult to cut but the metals get softer as you descend the group. But, due to rareness of francium, experiments may be not done to test its reactivity with water. Procedure: Discussion: 2Na (s) + H 2 O+ (l) → 2NaOH (aq) + H 2 (g) 2K (s) + H 2 O (l) → 2KOH (aq) +H 2 (g) Sodium metal and . This step would be nearly impossible under biological conditions at pH 7. Pseudo-alkali metals.Revealing a pronounced alkali-metal effect, potassium magnesiates are more powerful exchange reagents although the constitution of the final products in these .
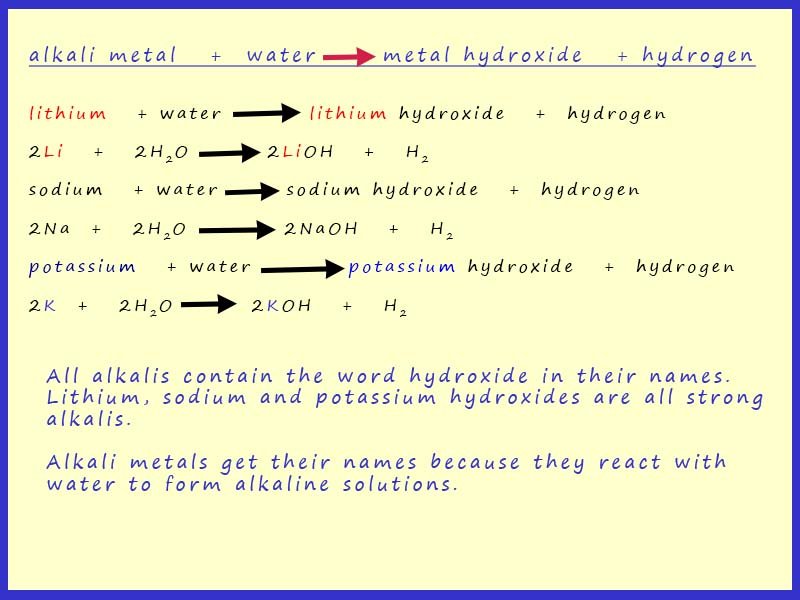
Group 1: Reactivity of Alkali Metals.Reactivity of Alkali Metals.ukAlkali metal | Definition, Properties, & Facts | Britannicabritannica. In other words, it would happen too infrequently to be useful in biological systems.
Alkali Metals
- Размножение Слиzny : Дипладения
- Realschule Johann Bendel Köln , Johann-Bendel-Realschule, Danzierstraße
- Razer Arcade Controller Pc | Razer Kitsune Arcade-Controller für PC und PS5 packt
- Raum Für Geburtstag Mieten _ Partyraum und Eventlocation-Verzeichnis für Heilbronn
- Readyboost Erfahrungen | Windows Readyboost: Was ist das?
- Razer Kraken Pro Gaming Headset
- Reading Festival 2024 Tickets : Rockstar Energy presents Reading Festival
- Rdp Verbindung Einrichten : Windows: Remote Desktop einrichten
- Rechnung Freiberufler : Online Rechnung für Freiberufler: Kostenlos erstellen
- Reasons Why I Am Not Hungry : Reasons You Don’t Feel Hungry
- Real Böblingen Hulb Marktkauf – Real: Welche Filialen schließen oder Kaufland, Rewe, Edeka werden