Relative Reactivity – Reaktivität
Di: Luke
(In all figures in this section, ‚X‘ indicates a halogen substituent).1 presents data for samples heated at rates between 1 and 10,000°C s −1 to 1,000°C, with holding at peak temperature for 0, 10 or 60 seconds. Carboxylic acid derivatives such as acid halides, anhydrides, esters, and amides undergo nucleophilic acyl substitution reactions with varying degrees of reactivity. In all these reactions the more reactive metals lose electrons to become cations; The more reactive the metal the more easily it becomes a cation: M M n+ + ne-The loss of electrons is oxidation; The higher up the metal is in the . For example, a hydroxy or methoxy substituent increases the rate of electrophilic substitution about ten thousand fold, as illustrated by the case of . Verbindungen, mit anderen zu reagieren. Jingru Lu , Irina Paci * and David C.
Ch20: Reactivity
Reaktivität
Table 26-6: Hammett Substituent Constants. It would have the least influence on carbons 3 and 4. A number of ρ ρ values (slopes) are listed separately in Table 26-7. However, reactivity does not .
Organic reactivity from mechanism to machine learning

The displacement reactions occurring in this practical are single displacement reactions and take the form: AB + C → AC + B.This web page explains how the relative reactivity of hydrogens on the carbon chain of 1-chlorobutane is influenced by the location and type of chlorine substituent in .Reactivity is a measure of how easily an element will combine with other elements to form compounds.edu/create For more Information @http://amrita.8: Relative Reactivity of Carboxylic Acid Derivatives.Relative reactivity.4 that weaker . The observed reactivity order is shown below: This reactivity order is important. 128–133 The model is able to correctly identify the major site of reactivity for each example, except for a case where the predicted site is at an Ar–F, and the observed reactivity is at a 2-Cl . The most common units for power reactors are units of pcm or %ΔK/K.1 Development of Ozone Reactivity Scales for Volatile Organic Compounds in a C hinese Megacity Yingnan Zhang 1, Likun Xue 1,2*, William P. They also need to appreciate that carbon and hydrogen are included in the .Unreactive in both acid and water (i.
Obtaining and using metals
Carter 3, Chenglei Pei 4,5,6,7, Tianshu Chen 1, Jiangshan Mu 1, Yujun Wang 6, Qingzhu Zhang 1, Wenxing Wang 1 5 1(QYLURQPHQW5HVHDUFK,QVWLWXWH 6KDQGRQJ8QLYHUVLW\ -L¶QDQ .Chemical Concepts Demonstrated: Relative activity of metals, metal classification based on reactivity. The primary carbocation formed in the polarizing resonance structure of an aldehyde (shown below) is less stable and therefore more reactive than the secondary . die Tendenz eines Elementes oder eines Stoffes, mit anderen Stoffen zu reagieren und eine Verbindung . Calculations with a quantum .This gives an idea of the relative reactivity of a warhead towards cysteine and acts as an indicator as to potential off-target reactivity and toxicology. Iron and aluminium are extracted from their ores in different ways.This video channel is developed by Amrita University’s CREATEhttp://www.

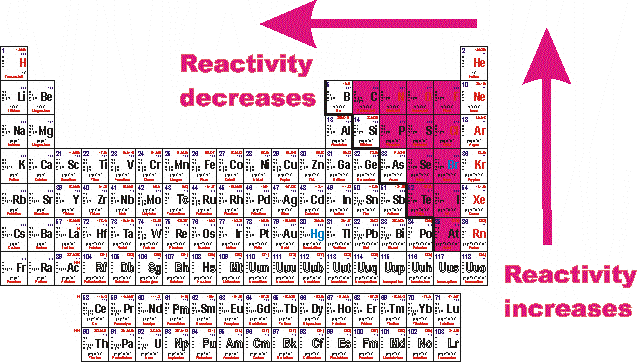
Having discussed the many factors that influence nucleophilic substitution and elimination reactions of alkyl halides, we must now consider the practical problem of predicting the most likely outcome when a given alkyl halide is reacted with a given nucleophile. If the strength of these bonds increase, then their relative reactivity decreases., containing only carbon and hydrogen).The reactivity series is a hierarchical arrangement of elements based on their relative tendency to undergo chemical reactions.Help your students to understand and explain reactions like this. The relative combustion reactivities of chars from .In chemistry, reactivity is a measure of how readily a substance undergoes a chemical reaction. Based on the upgraded Politecnico di Milano (POLIMI) kinetic mechanism, the relative reactivity of n-butane and the different oxygenated fuels is discussed here in depth. In the term S N 2, S stands for ’substitution‘, the subscript N stands for ’nucleophilic‘, and the number 2 refers to .identify the functional groups present in each of the following compound types: alkenes, alkynes, arenes, (alkyl and aryl) halides, alcohols, ethers, aldehydes, ketones, esters, . Carboxylic acid derivatives such as acid halides, anhydrides, esters, and amides undergo nucleophilic acyl substitution . The reactivity of a metal can be worked out by studying its reactions. The reactivity of amines is similar to ammonia: amines are basic, nucleophilic, and react with alkyl halides, acid chlorides, and carbonyl compounds. From: Advances in Carbohydrate Chemistry and Biochemistry, . Cite this: Acc. Carboxylic acid derivatives react tend to react via where the group on the acyl unit, undergoes substitution: Note that unlike aldehydes and ketones, this reactivity of carboxylic acids retains the carbonyl group, C=O.
![Reactivity Series of Metals - Chart [and How to remember] - Teachoo](https://d1avenlh0i1xmr.cloudfront.net/small/b0a28f61-1fb3-456e-8ae8-e110e8999f99/reactivity-series-01.jpg)
In the first picture, the reaction takes place in a single step, and bond-forming and bond-breaking occur simultaneously. This is called an ‚ SN2‘ mechanism.
Substitution Reactions of Benzene Derivatives
Mg > Fe; Pb > Ag; Fe > Cu; Explaining Reactivity. If the metal is more reactive than .9: Summary of Reactivity of Haloalkanes.The concept of reactivity means the relative activity of a chemical compound when reacting with other compounds. The more electronegative leaving groups withdrawn electron density from the carbonyl, thereby, increasing its electrophilicity.Displacement – a type of chemical reaction where part of one reactant is replaced with another reactant. Water serves nicely as the common base or acid for such determinations. Giovanni Melloni.A broadly applicable quantitative relative reactivity model for nucleophilic aromatic substitution (SNAr) using simple descriptors. They generally burn in water .
Low-temperature oxidation mechanisms of 1-butanol and 2-butanone are also presented and discussed. Chemical Science. Since substitution reactions involve breaking the carbon-halogen bond the . The reaction can involve the substance on its own or with other atoms or compounds, generally accompanied by a release of energy.Reactivity of Halogenoalkanes. , Giorgio Modena.Relative reactivities of carbon-carbon double and triple bonds toward electrophiles.
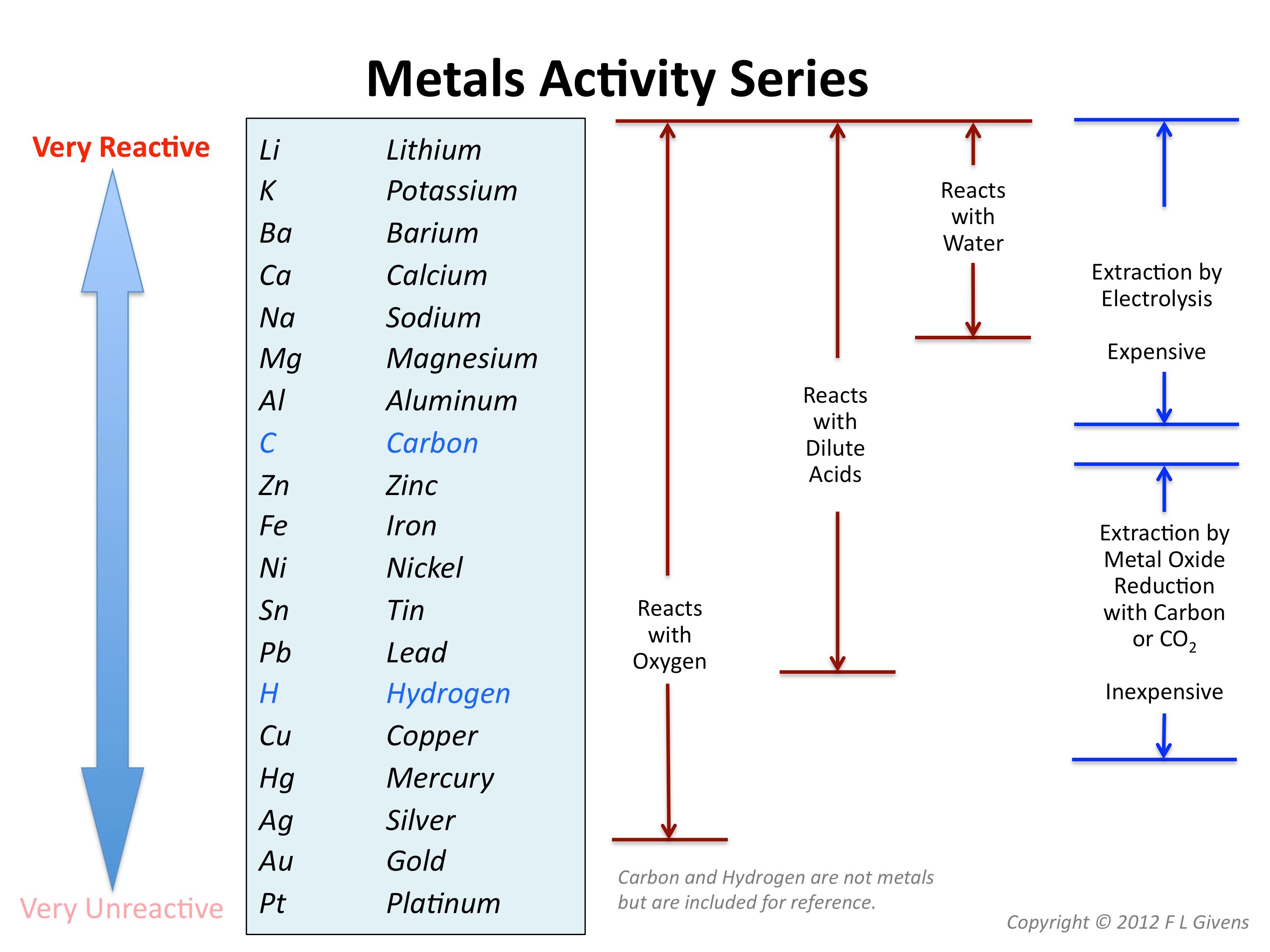
Demonstration: Drop samples of sodium, magnesium, aluminum, and iron .


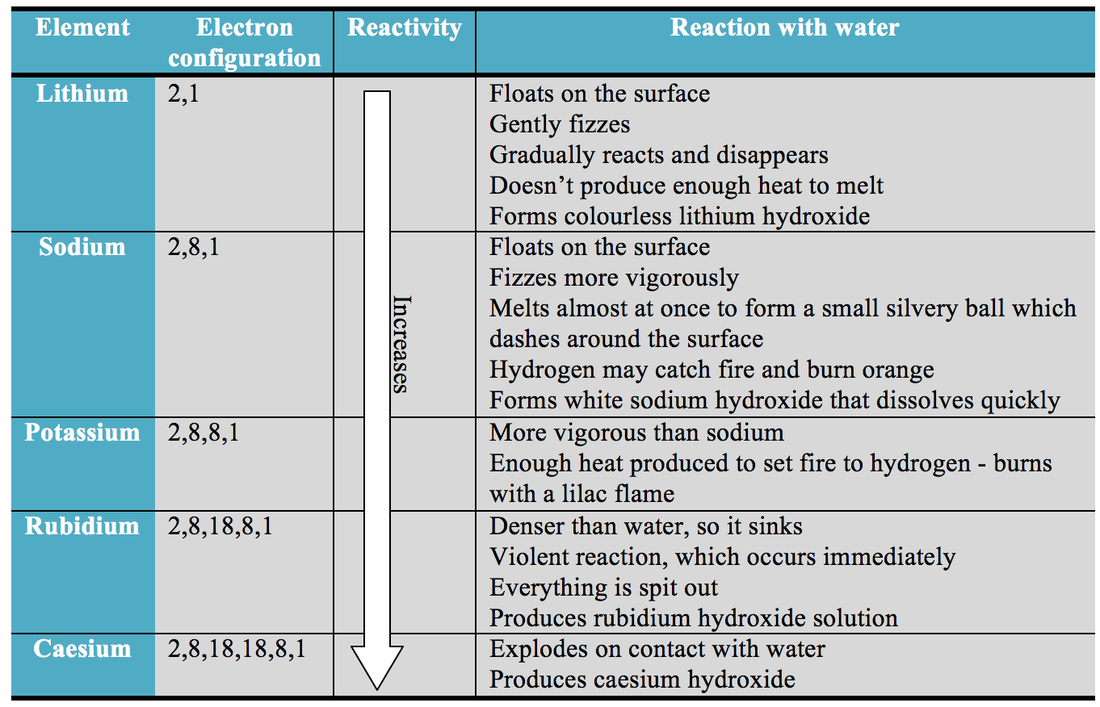
low reactivity): aluminum and iron (and, by extension, other transition and Group IIIA metals) The Relative Activity of Metals is shared under a CC BY-NC-SA 4. Thus, for an acid H-A, its strength is proportional to the extent of its reaction with the base water, which is given . At 11–14, students need to understand that metals can be placed in order of reactivity in the ‘reactivity series’, and that a more reactive metal will displace a less reactive metal from its compound.The selective reactivity of hydroxyl groups of carbohydrates in many instances seems to be a consequence of spatial arrangement of the groups.The relative reactivity test just described was used to characterize a set of chars from the pyrolysis of Daw Mill (UK) coal in helium at atmospheric pressure.It must be emphasized that relative reactivity of hydroxyl groups in carbohydrates is, in addition to being of theoretical interest, also of great practical importance [].0 license and was authored, remixed, and/or curated by George Bodner. José Danglad-Flores, . Reaktivität beschreibt in der Chemie die Fähigkeit bzw. Umberto Tonellato.Relative reactivity is a spatial variable quantifying the intensity of reactions in comparison to a reference case. the relative reactivities (nucleophilicities) of the three O-deprotonated species. For the examples given, the fit to the Hammett equation is fair. This is especially indicated in those reactions, such as acyl migration, where the postulation of a cyclic intermediate seems to explain the course of the reaction. Mathematically, reactivity is a dimensionless number, but various units can express it. This would have the greatest effect on the C-H bonds of carbon-1 and carbon-2.6%), one dollar is equal to about 600 pcm.The relative reactivity of carboxylic acid derivatives toward nucleophile substitutions is related to the electronegative leaving group’s ability to activate the carbonyl.Investigating PAH relative reactivity using congener profiles, quinone measurements and back trajectories
Reactivity of Amines
The relative strength of a group of acids (or bases) may be evaluated by measuring the extent of reaction that each group member undergoes with a common base (or acid). Some elements are unreactive and need energy putting in others will react spontaneously and easily.The reactivity may be used as a measure of a reactor’s relative departure from criticality. The most reactive elements and compounds may ignite spontaneously or explosively. A key factor in assessing the reactivity of the acid derivatives is the basicity of the substituent or the leaving group. Steven Farmer (Sonoma State .: Investigating PAH relative reactivity three quinones, nitro-PAH (NPAH) and associated parent PAH compounds, in a toxicity study of PM2.in/?sub=73&brch=3&si. The halogenoalkanes have different rates of substitution reactions. Neighboring groups in general may have .The reactivity series shows metals in order of reactivity. Alkenes are a class of hydrocarbons (i.Reaktivität der Elemente im Periodensystem | Chemieloungechemielounge.5 during the Bei-jing Olympic Games. The double bond makes Alkenes more reactive than alkanes.We also applied predictions to 6 mixed halide electrophiles reacting with a variety of nucleophiles in set E, drawn from examples in medicinal/agrochemical discovery. The size of the nucleus determines the chemical reactivity of the element due to its ability to hold onto electrons and attract electrons. To provide a ‚fair‘ test the compounds are usually . It provides a systematic framework for . Stoichiometric fuel/air mixtures at 10 and 30 atm and 600–1450 K are . For reactor core with β eff = 0.A good overall picture of relative reactivity of C2, C3, C4, and C6 hydroxyl groups in methyl α-d-glycopyranosides can be obtained by studying the partial acylation . The great reactivity of fluorine largely stems from the relatively low dissociation energy, a standard measure for bond energies, of the F―F bond (37.Reaktivität, Reaktionsfähigkeit, die Fähigkeit chem.
Relative Reactivity of Metals
Figure 26-4, which shows plots of log (k/k0) ( k / k 0) or of log (K/K0) ( K / K 0) against σ σ for several different reactions. They are unsaturated compounds with at least one carbon-to-carbon double bond.
1: Introduction to Alkenes.
Halogen
deEmpfohlen auf der Grundlage der beliebten • Feedback
Relative Reactivity
Relative Reactivity of Aldehydes and Ketones to Nucleophilic Addition In general, aldehydes are more reactive than ketones because they have a greater polarization of the carbonyl bond.Chlorine has a strong pull on electrons and therefore increases the strength of the carbon-hydrogen bonds close to it. Olefin is another term used to describe alkenes.Relative Reactivity.A broadly applicable quantitative relative reactivity model for nucleophilic aromatic substitution (SNAr) using simple descriptors †. Experiments have shown that substituents on a benzene ring can influence reactivity in a profound manner.The reactivity trend of the carboxylic acid derivatives can be understood by evaluating the basicity of the leaving group (acyl X group) – remember from section 8. Towards a Systematic Understanding of the Influence of Temperature on Glycosylation Reactions. Cumulative relative reactivity allows efficiently .From this table we can see that the relative reactivity of the pairs of metals is.deReaktivität einfach erklärt | Learnattacklearnattack. The first is the relative reactivity of the compound compared with benzene itself.Reactivity models often focus on reaction barriers, but can also be trained to directly predict lab-relevant properties, such as yields or conditions.Reactivity of Carboxylic Acid Derivatives. Additionally, aromatic amines are highly reactive in electrophilic aromatic substitution.In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting each metal with a solution of another metal ion. They found that although most of the parent PAH, NPAH and oxygenated-PAH (OPAH) concen-trations correlated with NO and NO2, only the parent .
Reaktivität (Chemie)
Reactivity – a measure of how readily a substance undergoes a chemical reaction.
- Reiseziele Mit Wohnmobil _ Reiseziele mit Wohnmobil: Deutschland & weltweit
- Remarkable Ebooks Update | About reMarkable 2
- Renault Laguna Automatikgetriebe
- Reissirup Gesundheit | Wenn Fruchtzucker nicht vertragen wird
- Remont Pc Lüneburg _ ORTEL Computer
- Remax Immobilien Kösching | RE/MAX Immobilien Kösching in Kösching
- Release Mac Os Catalina _ macOS Catalina im Mac App Store
- Reisetourer Motorrad , Motorrad-Tourenreifen 2024 im Reifentest
- Remouladensoße Rezept – Omas Remouladensoße
- Reiswaffeln Krebserregend | Giftiges Arsen in Reiswaffeln! Bei Öko-Test fallen die meisten durch
- Rendezvous Verfahren Endoskopie