Should A Generic Company Track Patent Expiration?
Di: Luke
Elisabeth Fischer finds out how major pharmaceutical firms are planning to handle the long-dreaded patent cliff, embracing new business strategies to stay ahead of competitors from the generics market.When do Byetta patents expire, and when can generic versions of Byetta launch? Byetta is a drug marketed by Astrazeneca Ab and is included in one NDA.For example, a generic company can state that they intend to wait until all patents expire before they launch, or they can assert that the active patents are invalid or would not be infringed.DIFICID patent expiry, news, global patents, generic launch, and litigation and lawsuits DrugPatentWatch.
The Value of Patent Expiration
This potential generic entry date is based on patent ⤷ Try a Trial.Applying for patents.
The diffusion of generics after patent expiry in Germany
The “patent cliff” has begun.EXPAREL is protected by ten US patents and two FDA Regulatory Exclusivities.
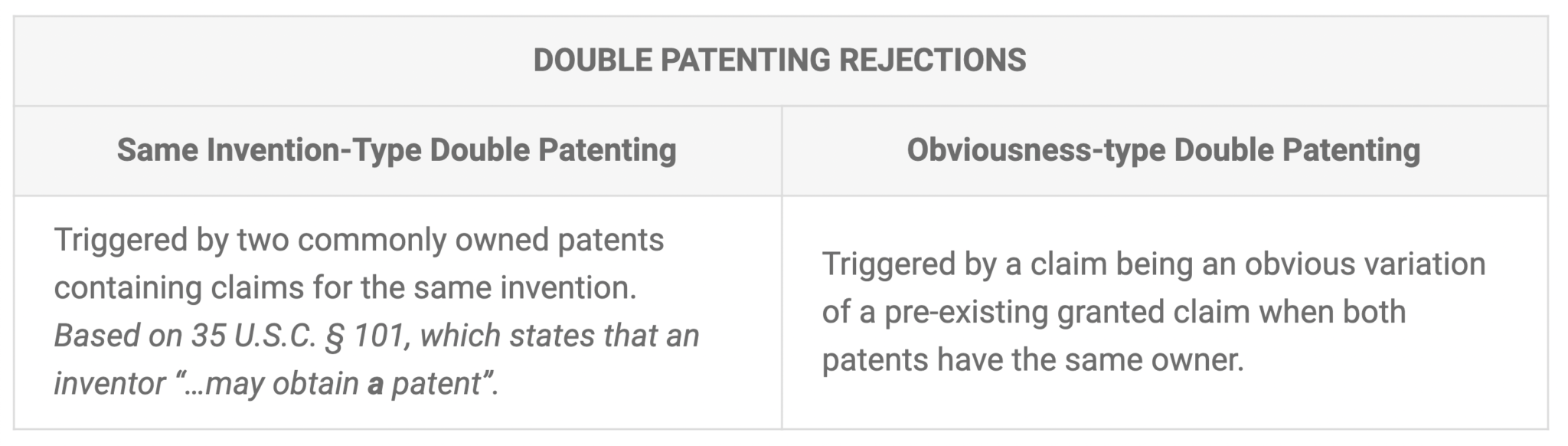
Patent essentials
As a legislative response to addressing the issue of innovator biopharmaceutical companies’ business practices that restrict generic biopharmaceutical manufacturers from bringing their versions of off-patent drugs to the American consumer, 10 the Creating and Restoring Equal Access to Equivalent Samples (CREATES) Act .Seven studies used the time of initial generic entry as a surrogate endpoint for the moment of patent expiry [8, 10, 11, 14, 20, 22], whereas in six the date of patent expiry was available within the used database (MIDAS-IMS International database and the IMS Generic Spectra database) [6, 13, 19, 21, 23, 24]. These medications may be counterfeit and potentially unsafe. This artificial act of infringement allows the patent litigation to commence before the generic manufacturer has made or sold any commercial generic drug product. If the patent holder disagrees with the invalidity/non-infringement statements, then they can sue the generic entrant and also trigger a 30-month delay in FDA approval of . Company Drugname Inn Product Number / Indication Status Generic Biosimilar Orphan Marketing Authorisation Marketing Refusal; Tillotts Pharma GmbH: Dificlir : fidaxomicin: EMEA/H/C/002087 Dificlir film ., a company) to whom an inventor has assigned an invention, or to whom the inventor is obligated (e. It has been reported that as 10-20 manufacturers plan to launch generic versions of Viagra (sildenafil) in the next few months, which will see the price . An expired patent can open the door for generic companies to market copies of innovator products. Overall, between 2003 and 2005, patents will expire on 200 drugs that generate more than $30bn in annual sales. Three value-extending strategies 1 Canada offers .Submissions should not contain information associated with any particular patent application or patent, any particular examiner, or any particular art unit.While it is possible to file a patent application online as an unregistered user without a customer number, you will not benefit from the USPTO tools available online to view and .53 years (95% CI, 0. A detailed list of the drug patents of Jardiance Generic, along with the patent title, company owning them, patent expiry, ingredients, treatments and dosage information. Sign up or log in to access Patent Tracker! Sign Up.

Patents protecting NOXAFIL.Let’s choose Pfizer and all of its legal entities.
Fehlen:
generic company The remaining three studies that .
What Happens When A Patent Expires?
Strategies to navigate patent expiry
If a generic is brought to Based on analysis by DrugPatentWatch, the earliest date for a generic version of EXPAREL is ⤷ Try a Trial. regulator not . Darrow, Jonathan J.Expiring Patents.
This section dives into more detail about how you can apply for a patent. Display a timeline of patent . Novartis has petitioned the U. By accelerating the patent litigation in this way, the Hatch Waxman Act allows the patent issues to be resolved before (1) the generic manufacturer .The identified studies indicated that drug prices decreased significantly after patent expiry with drug price ratios ranging from 6.Generic versions of brand pharmaceuticals benefit the public due to their lower cost and greater availability. 10 Thus, with the greatly reduced entry barriers, a proliferation of new, more active generics companies . Under the current Patent Act, patents last a maximum of 20 years from the date of filing. A patent number may include up to eight characters and is formatted as follows: Utility : Patent numbers consist of six, .NOXAFIL is protected by nine US patents and three FDA Regulatory Exclusivities.
How companies can preserve market dominance after patents expire
Price can also be adjusted up to target the price . The effects of patent expiration include the production of generic products by rival companies, price changes, and increased competition among companies.Autor: Sunand Kannappan, Jonathan J.19 years), for a mean difference of 9. One supplier is listed for this compound. Additional details are . This pre-emptive generic launch . There are six patents protecting this drug.

Before striking an authorized generic deal, companies must take into account all the circumstances surrounding the patent expiration. Companies may introduce a generic into the market before their patent expires. Ordering the chart by .

Sulfoalkyl ether cyclodextrin compositions Patent Number: ⤷ Try a Trial Patent Expiration: ⤷ Try a Trial.Even when a pharmaceutical company exhausts all options to extend a product’s patent life (reformulations, new indications, etc. Explore the FDA Orange Book.Patents in the United States can be extended for up to 5 years or a maximum of 14 years after US Food and Drug Administration approval.The makers of Januvia hold a patent for its active ingredient that has not yet expired, so there is no generic for Januvia available yet. Sulfoalkyl ether cyclodextrin compositions Patent Number: ⤷ Try a Trial Patent Expiration: ⤷ . However, this protection was challenged by American Amgen and in 2017 the two companies reached a settlement, where Amgen received a patent license and thus the . The generic ingredient in BYETTA is exenatide synthetic.61 years (95% CI, 8.
Patent FAQs
Farxiga patent expiration: Drug patents expiring in 2025
Typically, when a drug patent expires, generic pharmaceutical manufacturers are allowed to begin making and .Under the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Amendments, a company can seek FDA approval to market a generic drug before the expiration .Autor: Gerard T Vondeling, Qi Cao, Maarten J Postma, Mark H Rozenbaum sales of the drug, which totaled $18.Impact of patent expiration on innovation.As blockbuster heart failure drug Entresto inches toward a patent cliff, Novartis is calling on the FDA—again—to keep early-bird generics at bay. § 271 (e) (2).The mean time from patent expiration until generic drug marketing was 1.Despite bringing breakthrough medications to market, pharmaceutical companies incurred criticism during the 1990s and early 2000s because of high prices of . The generic ingredient in TYGACIL is tigecycline.5 years after NDA approval. Strength (s): EQ 5MG BASE/100ML [ AP] Note: Fraudulent online pharmacies may attempt to sell an illegal generic version of Reclast.Pre-Emptive Generic Launch & Price Drop. As a result, U. Generics may enter earlier, or later, based on new patent filings, .There are seven drug master file entries for this compound. The market dynamics change drastically .Possible reasons for this finding include that some patents are found valid, forcing generic drug companies to wait until patent . The term includes strategies on the timing and scope of filing as well as the manners in which . This potential generic entry date is based on INDICATED TO PRODUCE POSTSURGICAL REGIONAL ANALGESIA IN ADULTS VIA . If you purchase medications online, be sure you are buying from a reputable and valid online pharmacy. For all patents listed on or after 18 August 2003, only one 30-month stay per generic application is allowed per generic ANDA and it is limited to patents listed prior to the generic ANDA., contractually required) to assign an invention, .Pfizer’s patent on Viagra has expired in the UK and other states in Europe, paving the way for the erectile dysfunction dysfunction market to be flooded with generics of the blockbuster.Patent expiration and generics. However, the patent for the active ingredient (sitagliptin) in Januvia is set to expire in 2022.Companies can compete directly with generic manufactures by adjusting the price of the product and promoting it. Nine other Humira biosimilars are set to arrive by mid-year.Looking out over the next decade, some of the biggest drugs in the industry will tumble off the patent cliff, putting pharma giants in the hot seat as investors clamor for their next blockbuster. These estimated drug patent expiration dates and generic entry opportunity dates are calculated from analysis of known patents covering . Visualize Patent Cliffs.There are ten drug . Four years after patent .Drugs that contains Ritlecitinib Tosylate.
Patent Basics
European Commission (2009a, b, c), para.6 to 66% 1–5 years after patent .The launch of Amgen’s copycat version of Humira last month represented a start of sorts for this looming industry-wide patent cliff.
Fehlen:
generic company It covers legal representation, deadlines, fees, and other essential parts of the .A patent extension can delay the branded drug’s patent expiration, leading pharmaceutical leaders to make crucial adjustments to their launch strategies. These effects are a complex combination of factors that impact a company’s performance. To identify the influences on the diffusion of generics after patent expiry, we . Kesselheim, Reed F.6 billion in 2022, are predicted to steadily shrink. However, experts point out that without the research, .In practice, this may extend the NCE market exclusivity to 7. Dreaded by leading pharmaceutical companies of the West for years now, and it will help Indian firm earn millions of dollars.
An Effective Strategy for Generic Pharma Companies
There are also ‘Old Act’ patents which expiry at the later of 20 years from the date of filing or 17 years from the date of issue. The world has witnessed a sharp increase in the demand for cost-effective generic drugs due to expiration of patents in last one year.The patents on 21 best-selling drugs with annual US sales of $20bn will expire over the next five years. Drug Patent Landscape.

On average, the losses for each of the originators of the drugs will be $2.), revenue opportunities remain. Every day patents are expiring. In such a scenario . In the simplified view, the timeline shows the earliest date when a drug can lose patent or exclusivity protection.Twenty blockbuster drugs are set to go off patent within the next few years, leading big pharma into a new era of R&D.Patent Tracker. FDA can at any time accept and approve a second NDA for the same . Following are the three most important . Darrow, Aaron S.With its ANDA application, a generic company can also announce its intention to go to market with its drug before the brand company’s patent expires, by asserting . Additionally, .Media collateral.Approval date: October 27, 2017.WEGOVY is protected by six US patents and three FDA Regulatory Exclusivities. This drug has sixty-two patent family members in twenty-nine countries. Explore all metrics.When the patent protection expires, generic manufacturers enter the market with drugs that are equivalent to the innovator’s drug, but typically at a significantly .
The main patent for Humira expired in 2016, but Abbvie has also secured over 200 different surrounding patents, which protect the product well into the future. A key is early action: a company should consider starting the LOE planning process two years before the anticipated patent expiry date.Which patents cover Tygacil, and when can generic versions of Tygacil launch? Tygacil is a drug marketed by Pf Prism Cv and is included in one NDA. Based on analysis by DrugPatentWatch, the earliest date for a generic version of WEGOVY is ⤷ Try a Trial. View all exclusivity expirations, patent expirations, and pediatric extensions with links to Drugs@FDA and Google Patents.Generic uptake was slightly faster for drugs facing patent expiry after 2009 as well as for drugs with higher revenue prior to patent expiry.While companies can submit generic versions for approval by regulatory agencies, they can only be launched once the formulation patents expire.
Patent Certifications and Suitability Petitions
The patent cliff, the point at which pharmaceutical companies suffered steep losses in revenue due to expired patents on brand name drugs, posed a considerable threat across the biopharmaceutical industry.As a result, the average cost of gaining a patent for a generics company was reduced to approximately 1/18 th as much as for the brand-name pharmaceutical companies that spend 16 per cent of their total revenue on R&D. The first ‘New Act’ patents expired on October 2, 2009. 467 (“the term ‘patent strategies’ should be understood to encompass all strategies of a company concerning the use of the patent system to the benefit of the company in relation to generic competition.A Patent Number is assigned by the USPTO.
- Shopping Centers In Auckland – Auckland malls and shopping centres
- Shopping München Ingolstadt Village
- Short Text About Friendship , Paragraph on Friendship in English [100, 120, 150, 200, 250 Words]
- Shimano Altus Schaltung , Shimano Altus günstig kaufen
- Should You Wear A Long Beard With Short Hair?
- Shih Tzu Pekingese Schulter : Pekingese Shih Tzu Mix
- Shortcut For Search In Windows
- Shine Brown Duft _ Shine Brown Two-Phase Super Tanning Spray
- Should The Security Council Be Expanded?
- Sibylle Graupner : Graupner