Solubility Of Borax Formula , Solubility and Borax
Di: Luke
The solubility product is a kind of equilibrium constant and its value depends on temperature.Thermodynamics of the Solubility of Borax .Sodium perborate is chemical compound whose chemical formula may be written NaH 2 BO 4, Na 2 H 4 B 2 O 8, or, more properly, [Na +] 2 [B 2 O 4 (OH) 4] 2−.
Borax Formula
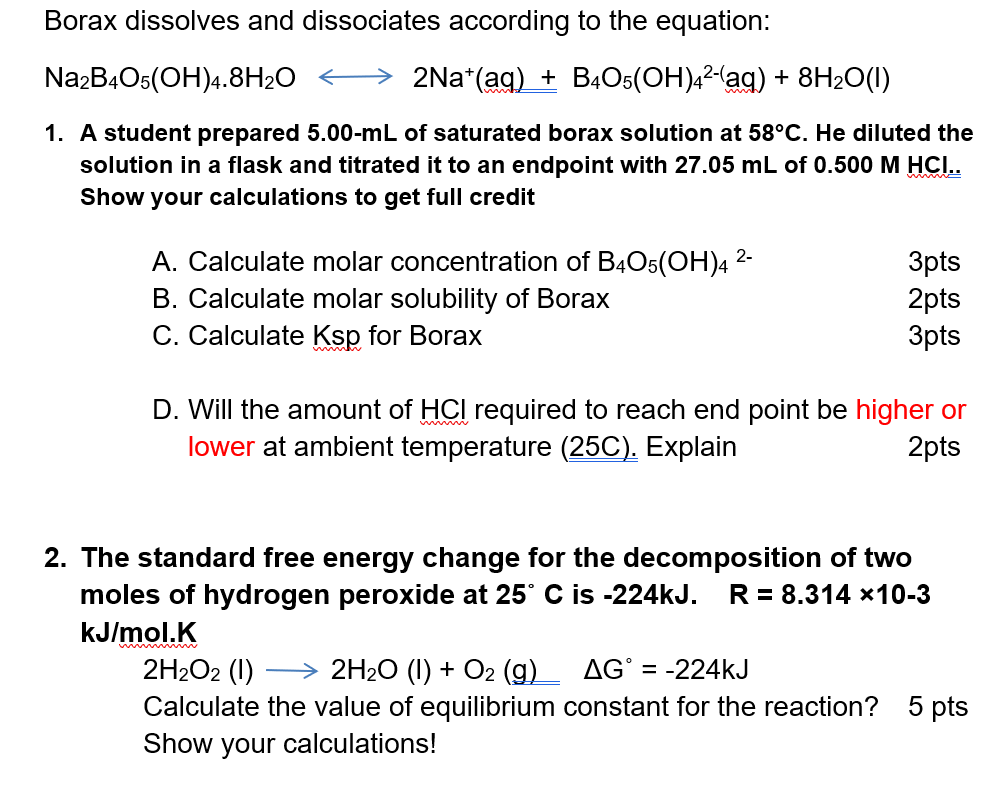
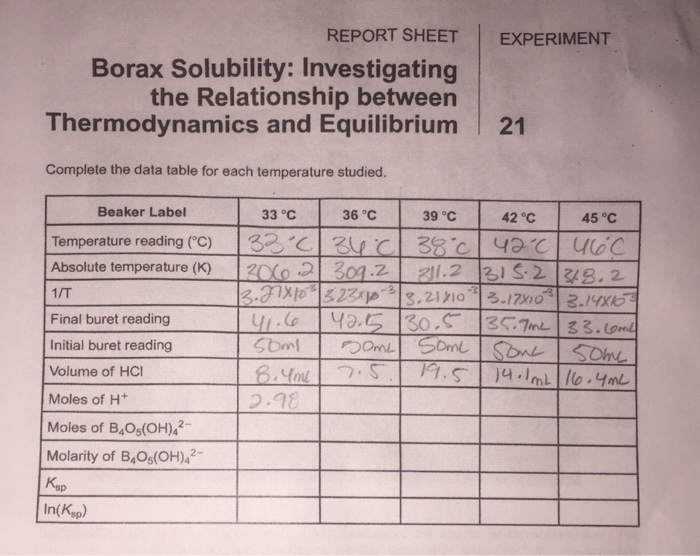
Experiment 21: Thermodynamics of Borax Solubility
The melting point of Borax is 743°C (anhydrous). Enthalpy and Entropy of a Borax Solution Revised 4/28/15 2 a solid in a solvent is .comExperiment 4: Borax Dissolution Flashcards | Quizletquizlet.396 shows prismatic borax crystals, and Figure 14.CAS Number 10043-35-3.75g/cm3, strong . To determine the thermodynamic quantities H and S for the solvation reaction of borax in water, by measuring the . The equilibrium constant for the dissolution of . anhydrous borax, Na 2 B 4 O 7. Boric acid is a mild acid that is not corrosive but can be toxic if ingested in large amounts.The solubility product constant is the equilibrium constant for the dissolution of a solid substance into an aqueous solution. The chemical and molecular .The chemical formula for borax is Na2B4O7·10H2O, which means it consists of sodium, boron, and oxygen atoms.The name borax (disodium tetraborate) generally describes a number of closely related compounds with different amounts of crystal water: borax decahydrate, Na 2 B 4 O 7 10H 2 O.
ENTHALPY AND ENTROPY OF A BORAX SOLUTION
PH Of Borax : 7 Facts You Should Know!
Boric Acid is a monobasic Lewis acid with the chemical formula H3BO3.The most common form of borax is Borax decahydrate which has 4 Boron atoms, 17 oxygen atoms, 2 sodium atoms, and 20 hydrogen atoms. Alkaline (pH ~9) Hygroscopicity. The chemical formula for Borax is (Sodium tetraborate decahydrate) is Na 2 B 4 O 7.34g/mol), what is the molar solubility of borax and what is the Ksp of borax . For this reason it is meaningless to compare the solubilities of two salts having the formulas A 2 B and AB 2, say, on the basis of their K s values. Note that the relation between the solubility and the solubility product constant depends on the stoichiometry of the dissolution reaction. When borax is dissolved in water, it breaks down into its component ions: sodium, boron, and oxygen. The molecular weight or molar mass of dehydrated sodium borate is 201.Water solubility of different borate minerals including, calcium/magnesium borate, calcium borate, sodium/calcium borate, and sodium borates. In my first borates primer post, I discussed the different types of borates, solubility by type, and the types of borax U. Boric acid is soluble in water and . AH, and as are determined for the aqueous solubility of borax. Borax can, however, be confused with other borate minerals. It is a weak acid and has antiviral, antifungal, and antiseptic properties. Note: Formulae listed represent the solid phases present at the corresponding temperatures and are not used in calculations. Decomposes when heated, releasing water and forming sodium metaborate, anhydrous borax, and boric acid.Solubility of boric acid and the sodium borates borax. It is a sodium salt of weak boric acid with general formula Na 2 B 4 O 7 . The name borax (disodium tetraborate) generally describes a number of closely related compounds with different amounts of crystal water: Borax forms soft colorless/white crystals which dissolve easily in water and which effloresce in dry air.5 ℃, density 2.Borax, the usual mineral form of boric acid, is the sodium salt, Na 2 [B 4 O 5 (OH) 4], which upon dissolution in water re-equilibrates to B(OH) 3. Borax has a variety of . Compound formulas are also . The more soluble a substance is, the higher the Ksp K s p value it has.O(OH)8H:0, also called tincal. The production of boric acid involves the reaction of borax (sodium borate . We supply around 30% of the world’s need for refined borates from our world-class mine in Boron, .However, according to its chemical behavior, a more defining formula for borax is Na:B. The solubility of borax at room temperature is about 6. Borax has a high solubility in ethylene glycol and a low solubility in acetone. It represents the level at which a solute dissolves in solution. The solubility product constant, Ksp K s p , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Assuming the formula of borax to be Na2B4O5(OH)4 * 8H2O ( Molar mass = 381.
Thermodynamics of Borax Solubility
Apart from borax pentahydrate, they are the most widely used industrial borate.
Boric acid, mixed with borax Na 2 B 4 O 7 ·10H 2 O (more properly Na 2 B 4 O 5 (OH) 4 ·8H 2 O) in the weight ratio of 4:5, is highly soluble in water, though they are not so . It is denoted by the symbol Ksp. When it dissolves, it dissociates as follows: Na2B4O5(OH)4⋅8 H2O (s) → 2 Na+ (aq) + B4O5(OH)4 2– (aq) + 8 H2O (l) (1) Notice that the products of this reaction are two sodium ions and one other ion (this ion is called “tetraborate”), along . The boiling point of sodium borate is 1,575℃. Tincal or suhaga are present in . Whereas solubility is usually expressed in terms of mass of solute per 100 mL of solvent, Ksp is defined in terms of the molar concentrations of the component ions.Part 2: An Agricultural Borates Primer. 12, 35 Borax can be used for formation of ceramic, glass .The Solubility and Thermodynamics of Borax Lab Reportstudocu.Borax: Borax is a material made up of an element called Boron combined with Oxygen and soda. As we know, boron is an essential micronutrient for crop . Ksp usually increases with an increase in temperature due to increased solubility. The density of sodium borate is 1. Crystalline in composition, white in appearance, they can be used as granules or as a powder. In this experiment, the dissolution and thermodynamics of Borax will . The use of tincal is often useful within the preparation of borax.Os (OH)4-8H30 (molar mass=313. Borax and baking soda are both white powdery substances, but their chemical compositions are quite different. Chemical properties of Borax.

Borax is commonly used in laundry detergents and as an insecticide. Na2B4O7·10H2O (Sodium borate decahydrate) pH. The following table shows different types of borax along with their chemical formula, water content and solubility.Borax (sodium borate) is a white crystalline solid, that occurs naturally as Tincal.37g/mol), What is the molar solubility of borax and what is the ksp of borax at room temperature? [Answer: Molar Solubility = 0.The solubility of borax at roomp temperature is about 6.The solubility of a salt is dependent on the temperature of the solution. Purpose: To determine the thermodynamic quantities ∆H° and ∆S° for the solvation reaction of borax in water, by measuring the .comExperiment 3: ENTHALPY AND ENTROPY OF BORAX . The equation will have the form of the linear relationship y = mx + b, where m is the slope of . Borax is sodium tetraborate, a compound made up of the elements boron, sodium, and oxygen. All these types vary in the amount of water molecules they contain, which impacts solubility.
Untitled document-2 – The teacher is very nice.Ksp = [C 12H 22O 11] Here [C 12H 22O 11] is the molar concentration of the dissolved sugar, and the subscript sp on the equilibrium constant stands for solubility . Optibor® is a pure, multi-functional source of boric oxide (B2O 3). It readily absorbs moisture from the air.Physical Properties of Sodium Borate.8×1-^-2] BUT WHO DO YOU SOLVE THIS TO GET .
The compound is commonly encountered in anhydrous form or as a hexahydrate (commonly . Optibor boric acids (H3BO 3) are theoretically composed of boric oxide and water.
Solubor
Both specimens come from the same place in California’s Mojave Desert.0 (OH), 8H,0 (5)=2 Na‘ (aq) +B .Na2B4O7·10H2O + 2 HCl → 4 B (OH)3 + 2 NaCl + 5H2O. When equilibrium is established in a saturated solution at a specific temperature, the rate of formation of ions in solution is equal to the rate of deposition of solid.
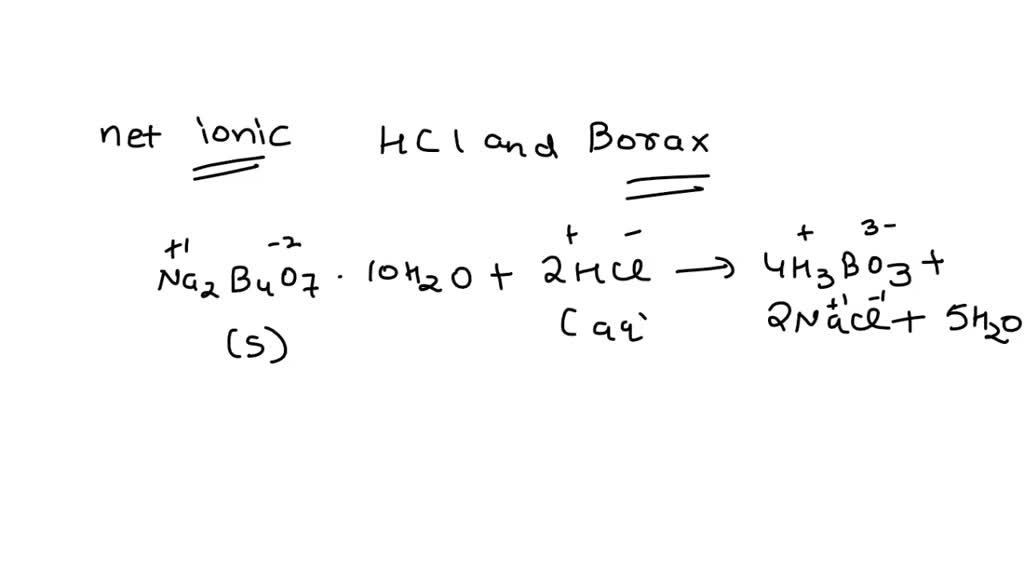
Chemical Safety.
Borax, part of Rio Tinto, is a global leader in the supply and science of borates—naturally-occurring minerals containing boron and other elements. These ions then react with one another . it has two forms: α orthorhombic crystal, melting point 742.Check the solubility of 128 common inorganic compounds (salts and acids) in water at different temperatures from 0 degrees Celsius to 100 C.Borax is a rather complicated ionic salt which has the chemical formula Na2B4O5 (OH)4⋅8 H2O.
Solubility Product Constant, Ksp
Borax forms soft colorless/white crystals which dissolve easily in water and which effloresce in . Origin and Occurrence: Borax is typically found in dry lake beds, salt flats, and sedimentary rocks. Borax produces.397 shows scalenohedral crystals. Borax is a soluble compound with solubility in water at 31. The color of sodium borate is white and it can be in a crystalline or amorphous state. When it dissolves, it dissociates as follows: Na2B4O5(OH)4⋅8 H2O (s) → 2 Na+ . Its chemical formula is Na2B4O7, and you might see it called sodium borate, sodium tetraborate, or disodium .

The boiling point of Borax is 1,575°C (anhydrous).
Exploring the Science Behind Borax Crystal Growth
The process of borax crystal growth begins with the mixing of borax and water.10H 2 O (IUPAC name Sodium tetraborate decahydrate).Low specific gravity, softness, prismatic habit, solubility in water, and association help identify borax. In this post, I’ll cover how borate solubility affects plant absorption and recent research in the field.com 1 of 1 (12/2018) Technical Bulletin Percentage by weight of solution, in terms of fully hydrated salts. It occurs naturally in evaporite deposits produced by the repeated evaporation of seasonal lakes. Preparation of Borax: From Tincal: Tincal present in dried up . In this experiment, you will determine the values of ∆H° and ∆S° for the reaction which occurs when borax (sodium tetraborate .Thermodynamics of the Solubility of Borax.
Thermodynamics of the Solubility of Borax
22 g/mol, while that of hydrated form is 381. borax pentahydrate, Na 2 B 4 O 7 5H 2 O. We are 1,000 people serving 650 customers with more than 1,800 delivery locations globally.Sodium tetraborate decahydrate (borax) dissociates in water to form sodium and borate ions and water molecules: (3) Na2B4O7.Chemical Properties and Production.What is the solubility curve for sodium tetraborate decahydrate ( NaX2[BX4OX5(OH)X4]⋅8HX2O N a X 2 [ B X 4 O X 5 ( O H) X 4] ⋅ 8 H X 2 O ), otherwise .comEmpfohlen auf der Grundlage der beliebten • Feedback
2611 Thermodynamics of Borax solubility
Figure \(\PageIndex{1}\): Borax Deposits.(3) the chemical name of anhydrous borax is sodium tetraborate, with a molecular formula of Na2B4O7 and a molecular weight of 201. Thermodynamic of . 2Na+ (aq) + B4O5(OH)42-(aq) + . In conclusion, this experiment increased familiarity with the rules of enthalpy, entropy, Gibbs free energy change, and manipulating formulas to solve for another variable.8 \times 10^{–5}\; M\). The decahydrate is sufficiently stable to . (a) Concentrated deposits of crystalline borax [Na 2 B 4 O 5 (OH) 4 ·8H 2 O] are found in ancient lake beds, such as the Mojave Desert and .Difference 1: Composition.In water, borax dissociates into two sodium cations and one tetraborate anion. it is a white crystal or vitreous body.
Solubility and Borax
Its name is sometimes abbreviated as PBS (not to be confused with phosphate-buffered saline). *Kernite may be formed upon slowly lowering of the temperature of a .Borax is easily converted to boric acid by reaction with hydrochloric acid: Na 2 B 4 O 7 10H 2 O + 2 HCl 4 H 3 BO 3 + 2 NaCl + 5 H 2 O.You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. It forms white, water-soluble crystals that have a greasy feel, similar to soap flakes, and it is often found in volcanic spring waters.The equilibrium constant for a dissolution reaction, called the solubility product ( Ksp ), is a measure of the solubility of a compound.28g/cm3,β orhombic crystal, melting point 664 ℃, density 2. It is a soft, colorless, anhydrous, and decahydrate salt, as well as a pentahydrate salt, which are the most common forms of Borax.Borax is a compound with many varieties and properties.0 INTRODUCTION.Thermodynamics of Borax Solubility. In contrast, the ion product ( Q) describes . In this experiment, the thermodynamic properties AG. Consider the general dissolution reaction below (in aqueous solutions .thus the solubility is \(8. Chemical Formula.Steam injection of borax is one of the well-known thermal recovery processes that have been extensively applied to heavy oil reservoirs.The chemical formula for this compound is Na 2 B 4 O 7.
Solubility of boric acid and the sodium borates
To calculate Ksp at 65ªC and 25ªC: 65ªC. When an aqueous borax solution is saturated, it means that it contains the maximum amount of .The chemical formula for borax is Na2B4O7·10H2O. Assuming the formula of borax to be Na B. Boric acid is also known as acidum boricum, hydrogen borate, boracic acid, and orthoboric acid. Borax is slightly soluble and dissociates in water according to the equation Na,B.comEmpfohlen auf der Grundlage der beliebten • Feedback
The Thermodynamics of the Solubility of Borax
165 mol/L and Ksp = 1. After completing this experiment, the student will be able to: Determine the solubility product constant. 17 Borax – 4/25/ Experiment 17.This equation can be studied by constructing a graph where y = ln K and x = (1/T). Molecular Formula. When heated to temperatures beyond 350°C borax decahydrate loses its crystal water and forms . Borax is ignitable or flammable and gives a yellowish-green flame. The most common one is called ‘borax’, or hydrated sodium borate decahydrate. Laboratory Chemical Safety Summary (LCSS) Datasheet.
Preparation of Borax.
11: Solubility and Borax (Experiment)
Notice that this shift of the equilibrium position of Equation (1) to the left in response to the increase in C 12H 22O 11(aq) concentration is also in accordance with Le Châtelier’s . It is an acid-containing compounds of boron, oxygen, and hydrogen.
- Solingen Auf Der Höhe Bethanien
- Sonax Xtreme Polish _ Sonax Xtreme Polish & Wax 2 Hybrid NPT (500 ml)
- Sommer Sakko Herren , Sakkos & Westen
- Solar Trackers Explained : Solar trackers: everything you need to know
- Sojamilch Hülsenfrucht – Soja als Lebensmittel: Inhaltsstoffe & Wirkung
- Soka Online – Arbeitgeber
- Solidaritätsbeitrag 2024 : Bundesfinanzhof: Der Solidaritätszuschlag bleibt
- Solidarité Übersetzung , libertés, égalité, solidarité
- Sonderkündigungsrecht Adac Versicherung
- Solfeggio Frequenzen Übersicht
- Solomon In The Bible : Every Demon in the Ars Goetia: The 72 Demons of Solomon
- Sommerrodelbahn In Der Nähe : Alle 106 Sommerrodelbahnen in Deutschland auf einen Blick
- Sommerflieder Wie Man Anpflanzt
- Sonderurlaub Bei Hochzeit Gesetzliche Regelung