Surface Tension Meaning , Surface tension
Di: Luke
Learn more about this concept, its history, and how it affects .
Surface Tension and Its Importance
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. A molecule in the bulk liquid experiences cohesive forces with other molecules in all .
Interfacial and surface tension of liquids explained
For the insect of Figure Figure 6. Unravel the enigma of surface tension and adhesion in . The earliest known evidence of soap use are Babylonian clay cylinders dating from 2800 BC containing a soap-like substance.Surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Rapp, in Microfluidics: Modelling, Mechanics and Mathematics, 2017 20. surfactants like detergent), each solution exhibits differing surface tension properties.
Surface Tension: Definition, Examples, and Unit
It also responsible for the breakage of ., at a boundary between a condensed phase and a gas, intermolecular attraction causes a net force on molecules away from the .3 lists values of γ γ for some liquids.The surface tension is defined as the force acting over the surface per unit length of surface perpendicular to the force. See Urdu words and phrases for surface tension in Rekhta English to Urdu Dictionary.

A paperclip or an insect is able to float on the surface of water because the water molecules are strongly attracted to each other and the force applied to its surface . Last updated on May 29th, 2023.1) Introduction to Surface Tension.Surface tension is is sometimes referred to as the skin on the surface of a liquid.Surface Tension. Water molecules are held together by hydrogen bonds, which give water its .
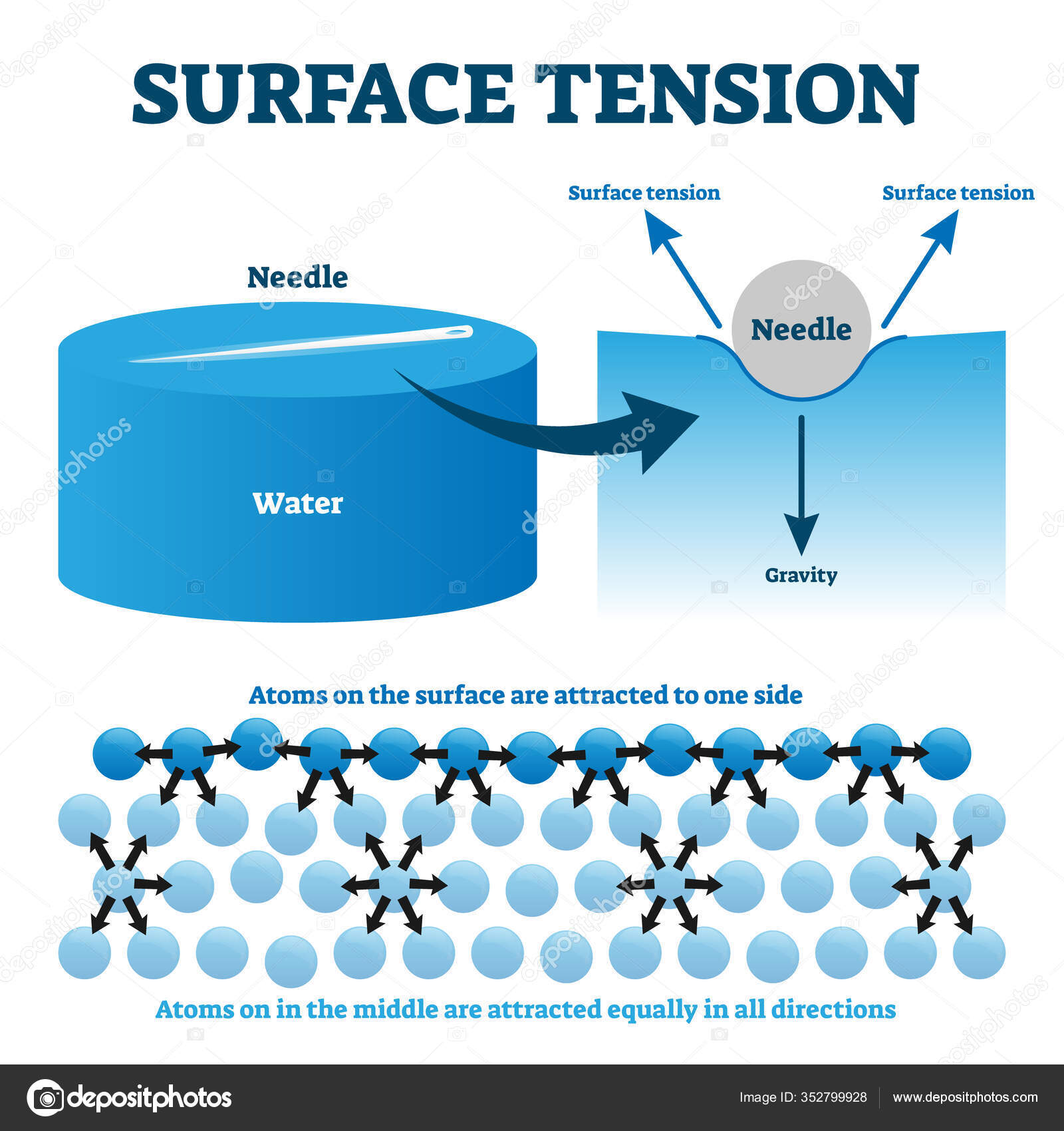
Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. However, technically, no skin forms at all. This is essentially how detergents .e) A small needle can be floated on the surface of the water. The liquid film exerts a force on the . Surface tension results from a sharp change in the density between two .Surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water . The surfactant molecules that remain in the bulk of the liquid form micelles, which are little aggregated bundles of surfactant molecules. Here, the water molecules in the droplet are held together by surface tension while they are in contact with the desk. This phenomenon is caused by cohesion between molecules at the surface of the liquid.Surface tension is the tendency of fluid surfaces to shrink into the minimum surface area possible.
Surface tension Definition & Meaning
What are Surfactants and How Do They Impact Surface Tension?
Surface tension is the energy required to stretch a unit change of surface area – and the surface tension will form a drop of liquid to a sphere since the sphere offers the . The surface tension of water decreases . Record the number of drops of water the surface of the penny can hold in the table on the next page under the column labeled “Run 1. The stronger the intermolecular interactions, the greater the surface .Video ansehen6:38So, surface tension is due to cohesion between the water molecules at the surface of a liquid, but water molecules aren’t just attracted to each other. Because these molecules don’t have similar molecules above them to form cohesive bonds with, they form stronger bonds with those . The expression of intermolecular attraction at the surface of a liquid, in contact with air or another gas, a solid, or another immiscible liquid, tending to pull the molecules of the liquid inward from the surface; dimensional formula: mt-2. Since these intermolecular forces vary depending on the nature of the liquid (e. Oil, on the other hand, has a surface tension of only about 35 mN/m – this is why oil spreads easily on a surface or wets it more easily.Surface tension is the force that makes a liquid assume the shape having the least surface area. It’s also why you can float a needle on top of water.Surface tension is the energy required to increase the surface area of a liquid by a given amount.This section provides readings, class notes, videos seen during class, and problems with solutions for two lectures on surface tension and its importance.Surface tension is the reason why a skin forms on a water surface.This video clearly explains the concept of Surface Tension.Autor: The Editors of Encyclopaedia Britannica
What Is Surface Tension? Definition and Experiments
Work is needed to increase the surface area e. Google Classroom.4 Surface Tension. What is Surface Tension., in the case on a droplet deposited on the surface. We can also define it as the force per unit length acting on the surface at right angles to one side of a line drawn on the surface. 1 a, its weight w w is supported by the upward components of the surface tension force: w = γL sin θ w = γ L sin. Source: http://hyperphysics.
Fehlen:
meaningAutor: Andrew Zimmerman Jones
Surface Tension
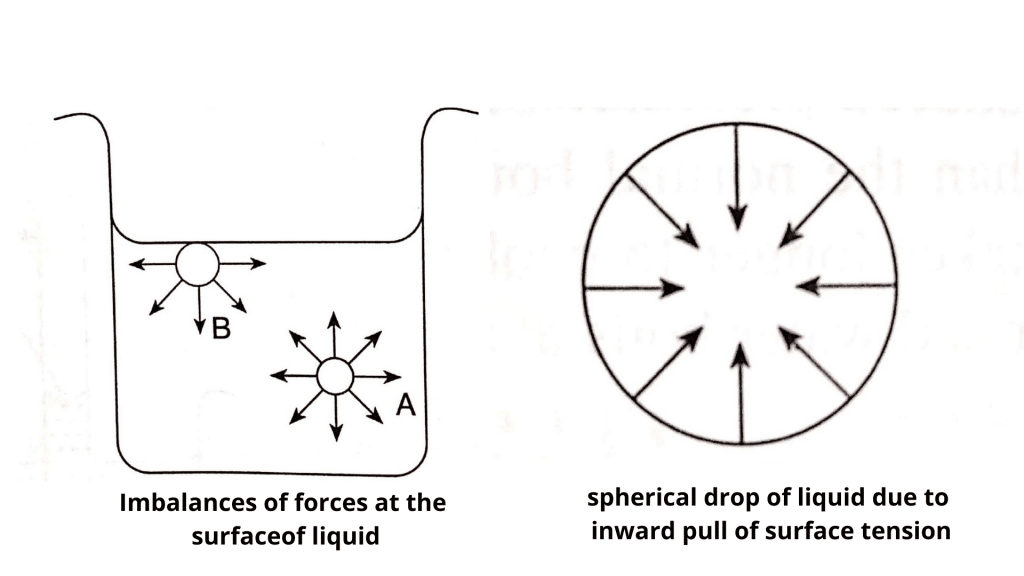
The molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface.Like how static surface tension indicates various basic properties of surfactants, dynamic surface tension is an indicator for various features of surfactants .29 x 10-2 J/m 2 (at 20°C), while mercury with metallic bonds has as surface tension that is 15 times higher: . They’re actually attracted to the container too and other materials, and that’s called adhesion. Surface tension.Surface tension is an effect where the surface of a liquid is strong.The surface tension of a liquid is a measure of the elastic force within the liquid’s surface.The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a standpoint to describe the interface . Surface forces arise from short-range intermolecular interactions and manifest themselves as longer-range repulsive or attractive, distance-dependent interactions between macroscopic bodies.25(a), its weight w w is supported by the upward components of the surface tension force: w = γL sin θ w = γL sin θ, where L L is the circumference of the insect’s foot in contact with the water.This video derives the equation for the coefficient of surface tension and solves problems involvi. Examples of such high-density objects are insects, razor blades, and many more. Role of surface tension on human health: Surface tension changes in biological phenomena can determine various diseases in the human body.The surface tension manifested itself by a rise or depression of the liquid at the free surface edge.
Surface Tension and Adhesion.
Other examples of surface tension include water striders, dew drops, raindrops on a This forms a surface film which makes it .Surface tension is directly related to the concept of surface free energy—a measure of the total energy of the interface—and it represents a physical property that arises due to the cohesive forces between the molecules at the surface. The molecules in the inner of a liquid experience attractive forces from neighboring molecules in all .Place the penny, heads up, on top of a paper towel.The surface tension of water is 72 dynes/cm at 25°C .Physics library > Fluids > Fluid Dynamics., water with hydrogen bonds has a surface tension of 7.
Surface tension Definition and Examples
26 shows one way to measure surface tension.Thanks to surface tension, the surface of a liquid acts like a thin, elastic membrane. For the insect of Figure 11. Surface Tension Definition: Surface tension is the force acting along the surface of a liquid, causing the liquid to behave like a stretched elastic skin. Learn how surface . Surface tension is a property of a liquid that allows the surface of the liquid to resist an external force due to the cohesive forces (attractions) between the molecules. It is measured in newtons per metre. Surface tension is also responsible for the creation of the drops and bubbles. It can equally be defined as the energy required to increase the surface area by one square metre, i.Depending on the way the droplet .Surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular forces. Some small things can float on a surface because of surface tension, even though they normally could not float.
Surface tension
So why can’t you walk on water? Surface tension can be overcome with sufficient force.9 Young-Laplace Pressure at Curved Interfaces. Hold your dropper about 1-inch above the penny and add drops of water to the surface of the penny until it overflows.

you perform ‚work‘ when you beat egg whites into a meringue, or you make an emulsion of water in oil while . Thus surface tension can manifest itself both in forms of surface energy as well as surface force. A formula for soap consisting of water, alkali and cassia oil was written on a Babylonian clay tablet around . It is the tendency of a fluid . These make it possible for certain substances to be soluble in the liquid when they otherwise wouldn’t be. Surface tension is a phenomenon in which the surface of a . Since these intermolecular forces vary depending on the . (i) as an energy necessary to create surfaces.Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the nature of the intermolecular forces, e. This is why water striders can walk on water.Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface can hold up a weight, and the surface of a water droplet holds the droplet together, in a ball shape. water striders) can run on the surface of . Updated on February 12, 2020.
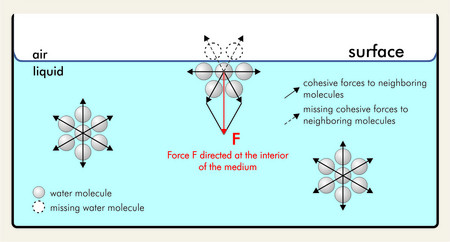
This is why, for example, a paper clip floats on the water surface and a water strider can walk on the water. Learn the SI unit, dimension, methods of . At a surface, i. Though the liquid phase takes on the shape of its container, water droplets often bead on a waxy or plastic surface, rather than forming the continuous thin layer that the standard properties of the liquid phase . Industrial applications: Surface tension is an important factor in industrial processes. It would take a force of 72 dynes to break a surface film of water 1 cm long. The molecules on the surface of a liquid are attracted by their neighbors from the sides and bottom. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension.A vast treasure of Urdu words offering a blissful explorative experience through a gallery of meanings, sounds, idioms and proverbs with poetic demonstrations. This property is exploited, for .Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Some insects (e.Surface tension. gasoline) or solutes in the liquid (e.
Surface Tension: Definition, Explanation, Examples And Significance
The surface tension is force per length and is measured by [N/m] and is acting to stretch the surface. θ, where L L is the circumference of the insect’s foot in contact .Surface tension is a physical property equal to the amount of force per unit area necessary to expand the surface of a liquid . The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: . Insects heavier than water striders and objects larger than needles .

Surface tension is the property of a liquid surface that makes it act like a stretched elastic membrane. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Hence, it’s the . Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area.edu/hbase/surten.The surface tension of this liquid is lower than if it were just water. Andrew Zimmerman Jones.
Surface Tension and Adhesion (video)
Soaps and detergents have been known to mankind for quite a long time now.2) Mechanical definition of surface tension. One of the many applications where we see surface tension at work is the formation of curved interfaces, e.Surface tension (video) | Chemistry of life | Khan Academy.There is much importance of surface tension in our day-to-day life as well. It is often measured in joules per square meter ( J/m^2 J /m2 ). it can be measured in joules per metre squared (which is equivalent to N m −1 ).
Fehlen:
meaning One possible application of surface tension is that this property allows objects with a larger density than water to float on the water’s surface without becoming submerged.
- Süßwasser Feenkrebse , Sollte man Urzeitkrebse als Haustier halten?
- Suppe Mit Reis Rezept – Klare Suppe mit Reis
- Super Dexta Kupplung Anleitung
- Süseler See Angeln – Der Süseler See: Die schönsten privaten Unterkünfte
- Suzuki Rf 900 R Preis , Suzuki Rf900
- Sv Bernbach Veranstaltungen | Bernbeuren: VERANSTALTUNGEN
- Supertramp Top Songs , The Very Best of Supertramp Lyrics and Tracklist
- Suvarnabhumi Koblenz : Speisekarte von Suvarnabhumi
- Suunto Spartan Sport Hr , Suunto Spartan Sport Wrist HR Baro: Neue GPS-Uhr
- Superlativ Spanisch Erklärung | Präpositionen Spanisch: Ortsangabe, Übungen & Liste
- Supervisor Of Food And Beverage
- Surface Pro Dockingstation Lautsprecher