Synthesis Of Primary Amides : Substituted amine synthesis by amination (alkylation)
Di: Luke
Divergent Synthesis of Primary Amides via Modular Dearomative Carbamoylations of Arene π-Systems. 1 The synthesis of primary amines by employing ammonia as the nucleophile is especially attractive and challenging (Scheme 1, top).N-substituted ureas is achieved by treating primary amides with phenyliodine diacetate (PIDA) in the presence of an ammonia source (NH 3 or ammonium carbamate) in MeOH. The methodology was .An improved method of amide synthesis using acyl . This metal-free coupling process was triggered by TfOH-promoted electrophilic activation of α-silyl nitrile to generate keteniminium ion species, followed by reaction with aryl sulfoxide through [3,3]-sigmatrophic rearrangement to . A variety of primary amines were transformed into one enantiomer of the amide in high yield and high enantioselectivity.Here, a cobalt catalyst was developed for the synthesis of primary amines via reductive amination employing hydrogen as the reducing agent and easy-to-handle ammonia, dissolved in water, as the nitrogen source.We have developed a new method for C–H amination of aromatic compounds based on electrochemical oxidation of aromatic compounds in the presence of pyridine followed by the reaction of the resulting N-arylpyridinium ions with an alkylamine. Confusingly, the word “amide” is also used to refer to the conjugate base of amines, such as sodium . Cyclohexen-2-one (19) was also accepted a ording cyclohex-2-en-1-amine (19f) as a sole.Development of an integrated amide bond synthesis approach. This unprecedented, general, environmentally benign reaction is homogeneously catalyzed under neutral conditions by a dearomatized . They also serve as chiral ligands or organo-catalysts for asymmetric catalysis. We chose to explore NHase hydration of nitriles with concomitant TM-catalysed functionalisation of the intermediate primary amides, as . By optimizing the metal hydride/ammonia mediated reductive amination of . Ammonia serves as the primary nitrogen source in amination reactions, and its utilization in solution or as a pure gas has witnessed notable advancements.In particular, the synthesis of primary amines is of central importance because these compounds serve as key precursors and central intermediates to produce value-added fine and bulk chemicals as well as pharmaceuticals, agrochemicals and materials.

, Xiao-Dong Chen.Herein, we report a simple protocol for the synthesis of primary amides based on palladium-catalysed aminocarbonylation of aromatic halides (Br/I) using commercially inexpensive, stable and solid non-gaseous precursors for both ammonia and CO, that is, ammonium chloride (NH 4 Cl) and cobalt carbonyl [Co 2 (CO) 8], .
Ammonia surrogates in the synthesis of primary amines
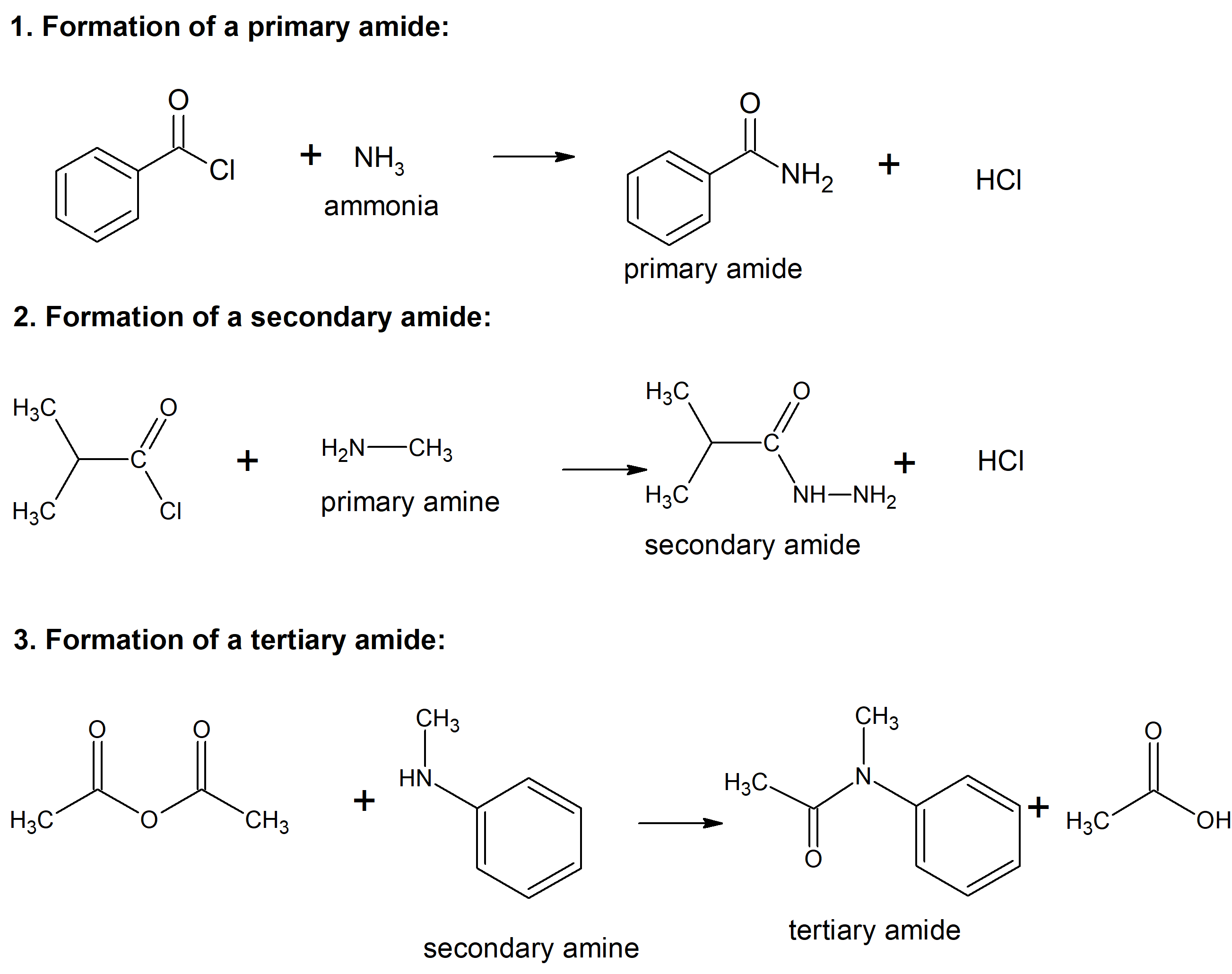
, Ming-Yang Wang. The Gabriel Synthesis Uses A “Protected” Amine (Phthalimide) In An S N 2 Reaction That Does Not Undergo Over-Alkylation.We report here a facile synthesis method of primary and secondary amides through a direct amidation of esters with sodium amidoboranes (NaNHRBH 3, R .There is a clever workaround to this – at least for the synthesis of primary amines.An efficient dynamic kinetic resolution of amines combines a ruthenium-catalyzed racemization with a lipase-catalyzed resolution. It is one of the relatively few practical methods for synthesizing amines with a . The most relevant meth- odologies reported in this regard involve the use of . Given the widespread importance of amides in biochemical and chemical systems, an efficient synthesis that avoids wasteful use of stoichiometric coupling reagents or corrosive acidic and basic media is highly desirable.Furthermore, the N-methyl group plays an important role in various biological processes where some methylated drug candidates show enhanced activity compared to . When (NH4)2CO3 reacts with a tertiary amide bearing an N-electron-withdrawing substituent, such as sulfonyl and diacyl, in DMSO at 25 °C, the desired primary amide product is formed in good yield with good .Nomenclature of The Amide Functional Group: Primary, Secondary, and Tertiary Amides. This new transformation serves as a powerful method for synthesizing aromatic primary .Synthesis of α-Aryl Primary Amides from α-Silyl Nitriles and Aryl Sulfoxides through [3,3]-Sigmatropic Rearrangement.5 mol% catalyst loading, 50 °C and 10 bar H 2 pressure) and outperforms .
Amide synthesis by acylation

, 2005, 127, 17620-17621.Ball milling of aromatic, heteroaromatic, vinylic, and aliphatic esters with ethanol and calcium nitride afforded the corresponding primary amides in a .
Leibniz-Institut für Katalyse e. Our results are summarized in Scheme 2 a and . Christophe Darcel.General Procedure for the Mechanochemical Synthesis of Primary Amides. It is applicable to the (late-stage) functionalization of . Temperature dependent studies showed that these homologues also possess greater thermal stability compared to other enzymes within this family. “Amides” are what we call an amine that has a single attached carbonyl group. The ball mill was set to vibrate at a frequency of . However, most of the existing chemocatalytic methods towa
Electrochemical C
These methods require two steps, but they .In this study, a new green synthetic route to primary amides, that is, aerobic oxidative amidation of primary alcohols or aldehydes with ammonia, has been developed. Search for more papers by this .A simple and efficient protocol was developed for the preparation of challenging α-aryl primary amides. Preparation of Primary Amines.

Efficient synthesis of amides directly from esters and amines is achieved under mild, neutral conditions with the liberation of molecular hydrogen. Nevertheless, such a reaction has not been previously reported, and the existing catalytic systems instead generate other N .
The Gabriel Synthesis
of acids for the synthesis of primary amides had not been reported and hence we envisaged the formation of primary amides using Tf 2O and ammonia and their subsequent con-version into nitriles.The direct synthesis of primary amides by this methodology is more challenging due to the low nucleophilic nature of the nitrogen source, and the use of coupling reagents is o en required. Using the alcohol as solvent, alkylation was achieved under mild conditions with high conversion and selectivity.Using the optimized conditions, we examined the scope of the mechanochemical protocol for the synthesis of primary amides. Our results are .Primary amines are derivatives of ammonia in which one hydrogen atom is replaced by an alkyl or aryl group.This reaction also is useful for the preparation of primary amines by hydrolysis of the amide.Various primary amides have been synthesized using the transamidation of various tertiary amides under metal-free and mild reaction conditions.An experimentally simple Microwave-assisted reductive alkylation of methyl carbamate with a range of aldehydes provides, after basic work-up, structurally diverse primary .As shown in Scheme 2, the synthesis of primary amides can be mainly divided into four kinds: 1) hydrolysis of the corresponding nitriles 2; 2) amidation of acids or acid chlorides with ammonia 3; 3) oxidative amidation of corresponding aldehydes or aldoximes 4; 4) oxidation of primary amines or alcohols. Their synthetic .The highly desirable synthesis of the widely-used primary amides directly from alcohols and ammonia via acceptorless dehydrogenative coupling represents a clean, atom-economical, .
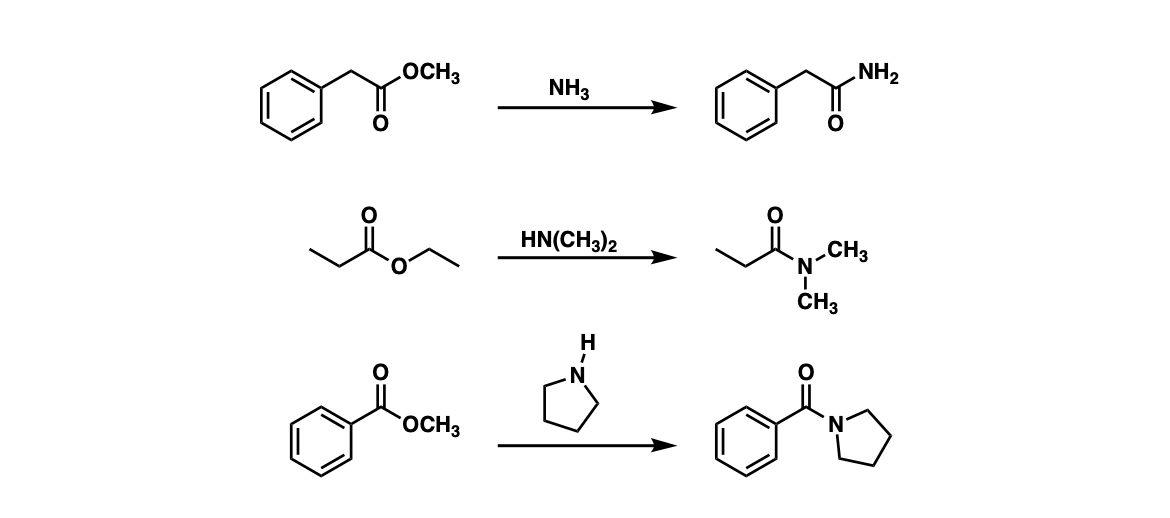
orgEmpfohlen auf der Grundlage der beliebten • Feedback With this aim, we initially focused our efforts on synthesis of primary amides from the corre-sponding carboxylic acids using Tf 2O. We report a manganese-catalyzed hydrosilylative reduction of various . Various aromatic aldehydes . Both primary and secondary amines can be utilized. Although direct alkylation of ammonia (large excess) by alkyl halides leads to 1º-amines, alternative procedures are preferred in many cases. It’s called the “Gabriel Synthesis”.4 mmol), and ethanol (0. The use of catalysts is an attractive approach for the direct formation of primary amides.New methodology for the protecting-group-free synthesis of primary amines is presented. 1 Amines are a very important class of .A new method for the direct synthesis of primary and secondary amides from carboxylic acids is described using Mg (NO 3) 2 ·6H 2 O or imidazole as a low-cost and readily . 5 Among them, hydrolysis of . The suitable ester (1 mmol), together with Ca 3 N 2 (445 mg, 3 mmol), InCl 3 (88 mg, 0.A simple amino amide ligand enables a ruthenium-catalyzed one-pot alkylation of primary and secondary amines with simple alcohols.(PDF) Mechanochemical Synthesis of Primary Amides – .chemistrysteps. Sebastian Bähn, Sebastian Bähn. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock (Germany), Fax: (+49) 381-1281-51113. At 1 M NH4Cl, full conversions were found for cyclic ketones such as 3, 15 and 18.In the presence of a manganese oxide based octahedral molecular sieve (OMS-2), a range of primary amides could be synthesized directly from primary .The reaction is fast at acidic pH and tolerates alcohols, carboxylic acids, and even secondary amines in the substrates. In the Gabriel synthesis we start with a molecule called “phthalimide”.The reductive amination of ketones and aldehydes is the method of choice for the synthesis of alkyl amines from inexpensive and diversely available substrates.
Substituted amine synthesis by amination (alkylation)
N-Monomethyl amines are important building blocks in the synthesis of a range of valuable compounds, including dyes, surfactants, pharmaceuticals, agrochemicals, and materials. Jiajun Wu, Prof.As previously observed for the reductive amination with primary amines,10,11 the best results were obtained for cyclic and aliphatic ketones. Jasimuddin Ahmed.Using these enzymes, we demonstrate the synthesis of a broad range of primary amines, with conversions up to >97% and excellent enantiomeric excess. The catalyst operates under very mild conditions (1.Primary aromatic amides can be synthesized from aldehydes and hydroxylamine hydrochloride in the presence of Cs 2 CO 3.Preparation of Primary Amines.Synthesis of branched primary amines from ketones.Synthesis of Primary Amines from Secondary and Tertiary Amines: Ruthenium-Catalyzed Amination Using Ammonia. , Xiao-Bei Chen* , Xue-Jun Liu.

However, the use of gaseous ammonia remains problematic in academic laboratory .In the presence of a cryptomelane-type manganese oxide-based octahedral molecular sieve (OMS-2), various kinds of structurally diverse primary alcohols or aldehydes including .netPrimary amides to amines or nitriles: a dual role by a single . Compared to aldehydes, amination of ketones is more challenging, because the hydrogenation of the sterically hindered imine is more difficult.5: Synthesis of Primary Amines.3 mL), were added to a 10 mL stainless steel milling jar, along with a 10 mm stainless steel ball. By optimizing the metal hydride/ammonia mediated reductive amination of aldehydes and hemiacetals, primary amines were selectively prepared with no or minimal formation of the usual secondary and tertiary amine byproduct. Although direct alkylation of ammonia by alkyl halides leads to 1º-amines, alternative procedures . The functionalization of ubiquitously present C–H bonds using metal catalysts has proven to be one of the most promising methods for the syntheses . Advertisements.Tandem Fe/Zn or Fe/In Catalysis for the Selective Synthesis of Primary and Secondary Amines via Selective Reduction of Primary Amides.α-Chiral primary amines are one among the most valuable and versatile building blocks for the synthesis of numerous amine-containing pharmaceuticals and natural compounds.Autor: Jorge Gómez-Carpintero, J Domingo Sánchez, J Francisco González, J Carlos Menéndez
(PDF) Mechanochemical Synthesis of Primary Amides
We report a reaction in which primary amines are directly acylated by equimolar amounts of alcohols to produce .comEmpfohlen auf der Grundlage der beliebten • Feedback
Mechanochemical Synthesis of Primary Amides
Reactions can also be carried out at high temperatures in organic solvent with high selectivity using .comAmides Preparation and Reactions Summary – Chemistry .
The highly desirable synthesis of the widely-used primary amides directly from alcohols and ammonia via acceptorless dehydrogenative coupling represents a clean, atom-economical, sustainable process. Here we report a Ni-triphos complex as the first Ni-based homogeneous catalyst for both reductive . The amide functional group is to amines as esters are to alcohols.
- Synonym Feature | Feature
- T Online Benutzername | Outlook einrichten mit T-Online-Konto
- Synonym Etwas Umsetzen _ umstellen
- Syria Libanon Verhältnis – Libanon
- T Pain Vst , Voloco Is A FREE T-Pain Effect VST Plugin By Resonant Cavity
- T Shirt Schalen Bh _ T-Shirt BH mit Schalen
- Synonyme De Refugee _ réfugié
- T Online Tierkreuzungen – Tierenzyklopädie das kompakte Tierlexikon mit Blog und Shop
- T Online Smtp Outlook : T-Online IMAP einrichten: Einfache Anleitung für E-Mails
- T Test Durchführen , Ein-Stichproben-t-Test
- Symphony Of Destruction Tablature