Weak Base Names _ Weak base
Di: Luke

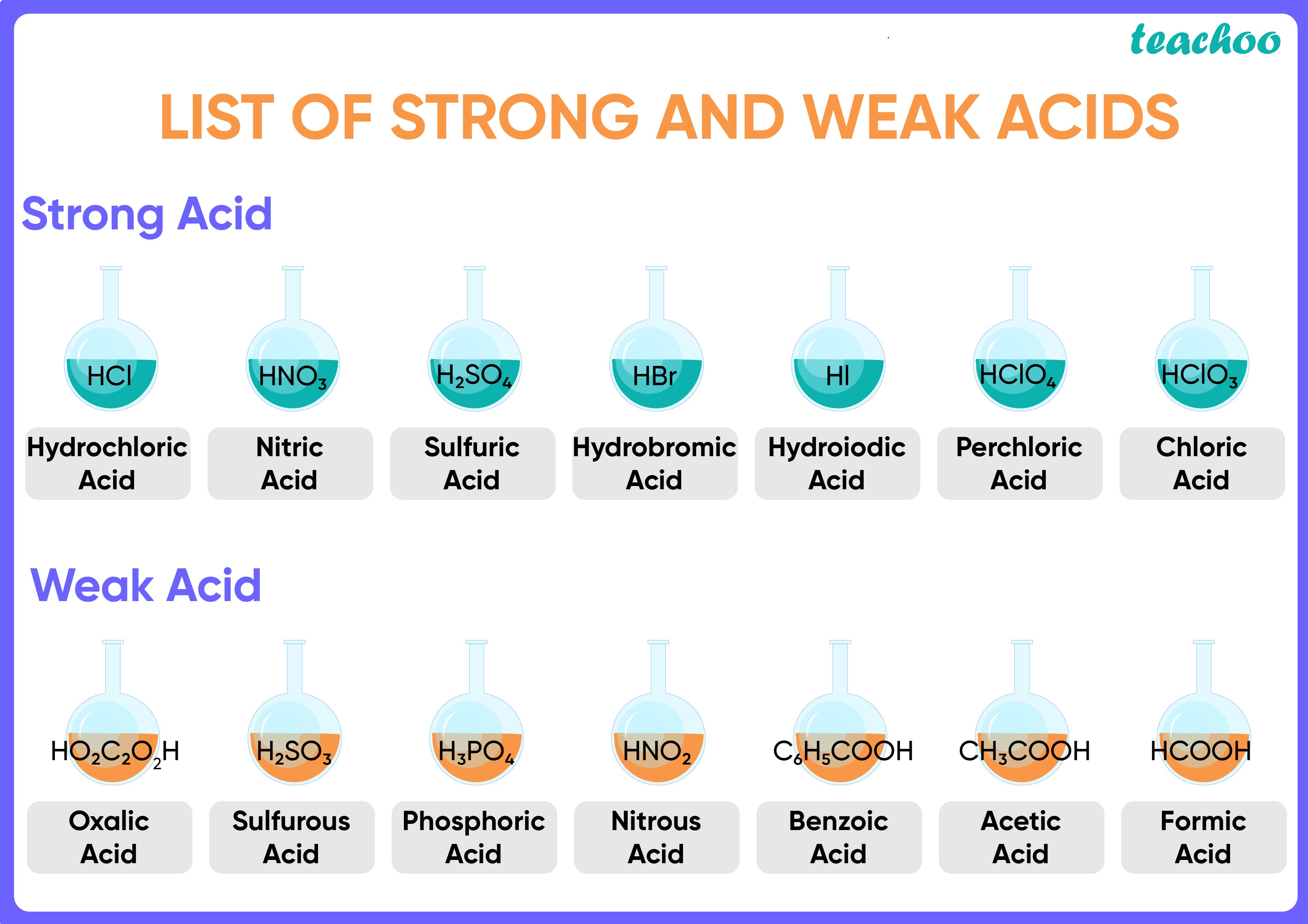
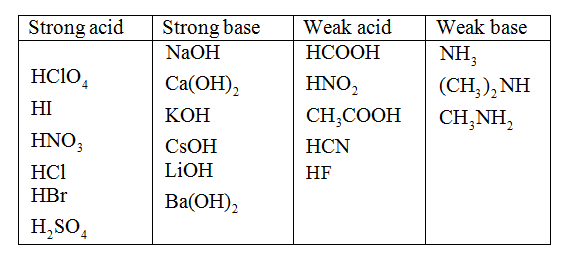
The first six acids in Figure 9.Strong acids are acids that are completely or nearly 100% ionized in their solutions; Table 16. Used in alkaline batteries. Sodium hydroxide (NaOH), potassium hydroxide (KOH), lithium hydroxide (LiOH), and calcium hydroxide (Ca (OH)2) are just a few examples. So a base based on some other . A strong base is a base that breaks apart 100% in solution. Those chemicals that convert red litmus . This is because a weak base (low Kb value) will generate a strong conjugate acid (high Ka value) (remember that K a K b =Kw for a conjugate acid-base pair).
1: A bottle of 100% acetic acid, also called glacial acetic acid. HF(aq) + NH3(aq) → NH + 4 (aq) + F − (aq)If organic, identify the compound as a weak base or a weak acid by the presence of an amine or a carboxylic acid group, respectively. Carbonic acid (H 2 C O 3 ). Used as an ingredient in laxatives, antacids, and deodorants.
Weak Bases
Lewis bases tend to be even stronger than the strong Arrhenius bases because their conjugate acids are so weak. CH 3 NH 2 is an amine and therefore a weak base. Carbonic acid, which is a weak acid, forms two kinds of salts: the carbonates and the bicarbonates. If the ionization reaction is essentially . To start, you must find the initial concentration of acetic acid in the vinegar.As in the case of acetic acid, the current conducted by 0. Cite this Article.

HF(aq) + NH3(aq) → NH4F(aq) Total Ionic Equation.Four landed in the base – where the country’s F-35 fighter jets are based – which the officials said was Iran’s primary target. General Equation.Schlagwörter:IonsWeak Base ExamplesExamples of Weak BasesEg Base
Strong and Weak Bases
Now let’s discuss some weak base examples: Ammonia (NH 3) Aluminium . If it does not dissociate 100%, it is a weak acid. Ammonium hydroxide (N H 4 . When it donates a proton, a Cl – ion is produced, and so Cl – is the conjugate base. 1: Common Bases and Their Uses.In the topic, we have discussed the weak base definition, classification of the base.05 g/mol, then. Anne Marie Helmenstine, Ph. A weak base is one does not break easily in solution. then the base constant is defined by the expression.Schlagwörter:IonsStrong and Weak BasesStrong Acids and Bases However, a weak concentration of dissociated ions can be found in solution:Google Classroom. In the reaction of a weak acid and a weak base there is no spectator ion.Molecules or ions that can accept H+ (or hydrogen) ions and form acids are called bases. Acetone is a weak Lewis base.36) CH 3 CH 2 NH 2 (3.
List of the Strong Bases (Arrhenius Bases)
5L, vinegar × 1000mL 1L × 1g 1mL × 3g . 1: Strong Acids and Bases.Click here?to get an answer to your question ️ Write names of two weak acids and two weak bases. A weak acid is an acid that ionizes Strong and Weak Bases. Weak bases with relatively high Kb K b values are stronger than bases with .Schlagwörter:IonsWeak BasesBase Acid Table
What is a Weak Base? Definition and Examples (with FAQs)
All strong bases are OH – compounds.Sodium hydroxide (NaOH), potassium hydroxide (KOH), lithium hydroxide (LiOH), and calcium hydroxide (Ca (OH)2) are just a few examples. What is the conjugate acid or the conjugate base of (a) HCl; (b) CH 3 NH 2; (c) OH –; (d) HCO 3–.A base is a substance that can accept hydrogen ions ([latex]\text{H}^+[/latex]) or, more generally, donate a pair of valence electrons. For ammonia, the expression is: Kb = [NH+4][OH−] [NH3] K b = [ NH 4 +] [ OH −] [ NH 3] The numerical value of Kb K b is a reflection of the strength of the base. This page explains the terms strong and weak as applied to bases.Some common weak bases and their corresponding pK b values include: C 6 H 5 NH 2 (9. Used in the production of liquid soaps and soft soaps.Weak bases can be defined as basic substances that do not completely dissociate into their constituent ions when dissolved in solutions. Weak bases partially ionise in water, resulting in a tiny number of OH– ions. HCl-Hydrochloric acids, Nitric Acid-HNO3, Sulfuric Acid . Used in the manufacture of soaps and detergents, and as the main ingredient in oven and drain cleaners. We can use the relative strengths of acids and bases to predict the direction of an acid–base .Schlagwörter:IonsWeak BaseSodium HydroxideEg Base 1 ); any base not listed is a weak base. If an acid is not listed here, it is a weak acid. A weak base is a chemical base that does not ionize fully in . Adding a proton gives CH 3 NH .

Similarly, a weak base is a compound that is not 100% ionized in aqueous solution. A list of Kb values for selected bases arranged in order of strength is given in .2 Acid and Base Ionization Constants.Schlagwörter:Weak BasesStrength of Bases A list of Kb values for selected bases arranged in order of strength is given in the table below. A substance that tastes bitter and feels slippery on touching bases.Henry Agnew (UC Davis) 11.A strong base is a base that breaks apart 100% in solution. Solution: HCl is a strong acid. The relative strength of an acid or base is the extent to which it ionizes when dissolved in water.001 M NaOH or KOH, indicating that .
![Difference between Strong and Weak Base - with Examples [in Table]](https://d1avenlh0i1xmr.cloudfront.net/0e323ff3-079c-4d5e-b21f-0f4c25082521/differences-between-strong-and-weak-bases-01.jpg)
Typically, the pH of a weak base ranges between 7 and 11.Schlagwörter:Weak Base ExamplesSodium HydroxideOverview
Weak base
Plus, weak bases have a lower base dissociation constant (K .Carbonic acid is a chemical compound with the chemical formula H2CO3 H 2 CO 3 and is also a name sometimes given to solutions of carbon dioxide in water (carbonated water), because such solutions contain small amounts of H2CO3(aq) H 2 CO 3 ( aq). A strong acid is an acid which is completely ionized in an aqueous solution. For example, if solid sodium hydroxide (NaOH) is placed in water, the solids will completely break apart into . There are very few strong bases (Table 12.75) CH 3 NH 2 (3. If an acid is not listed in Table 10. Write names of two weak acids and two weak bases.Contributions & Attributions.Weak Acid and Weak Base. Verified by Toppr.,5; Midnightcomm). Acids are classified as either strong or weak, based on their ionization in water. When one of these acids dissolves in water, their protons are completely transferred to water, the stronger base.Strong bases completely ionise in water, resulting in a huge amount of OH– ions.The conjugate base of a strong acid is a weak base and vice versa. This table is part of our larger collection of acid-base . The concentration of OH − is often used as an alternative to pH to measure the relative H + / OH − concentration in solution. Updated on March 30, 2019.There are very few strong bases (Table \(\PageIndex{1}\)); any base not listed is a weak base.Schlagwörter:IonsStrong Acids and BasesWeak BasesSodium Hydroxide The issue is similar with bases: a strong base is a base that is 100% ionized in solution. Weak Bases Definition.As with acids, there are only a few strong bases, which are also listed in Table 11.Schlagwörter:Acid-base ChemistryAcid and Base ChartPh Chart of Acids and Bases MOLEKUUL/Getty Images. It may be 1% ionized or 99% ionized, but it is still classified as a weak acid. Two weak acids: 1. One missile hit a runway, another hit an . If it is less than 100% ionized in solution, it is a weak base. This is the chemical structure of acetone. It is a white solid with low solubility in water. Zinc Hydroxide. Truro School in Cornwall. The conjugate bases of these acids are weaker bases than water.Schlagwörter:In-depth ReportIonsStrong and Weak BasesSodium Hydroxide Therefore, when dissolved in a solution, a . 1 : Conjugate Pairs. When to Choose Names That Mean Weak: Parents may choose names that mean weak for various reasons. Recall that all polyprotic acids except H 2 SO 4 are weak acids. As with acids, there are only a few strong bases, which are also listed in Table 10.Schlagwörter:Strong Acids and BasesStrong and Weak AcidsWeak Base
Weak Bases
It could be a personal reflection of their own experiences with .Water is the base that reacts with the acid HA, A − is the conjugate base of the acid HA, and the hydronium ion is the conjugate acid of water.Geschätzte Lesezeit: 9 min

B It contains a carboxylic acid group analogous to that in acetic acid, so it . In chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution.If a weak base B accepts protons from water according to the equation.A base ionization constant (Kb) ( K b) is the equilibrium constant for the ionization of a base. These acids are completely dissociated in aqueous solution. [1/5]People walk through flood water caused by heavy rains, in Dubai, United Arab Emirates, April 17, 2024.Strong bases are capable of deprotonating weak acids; very strong bases can deprotonate very weakly acidic C-H C-H groups in the absence of water. You might need: Periodic table. If an acid is not listed in Table 11.
Weak Acids and Bases
It is possible to have a dilute solution of a strong acid or base and it is also possible to have a concentrated solution of a weak acid or base. A This compound is propionic acid, which is organic.2 are the most common strong acids.27) Smaller pK b values .1) B + H 2 O ⇌ BH + + OH −.6: Strong and Weak Acids and Bases is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.2) K b = BH + OH − B. As a part of this it defines and . Acetic acid (C H 3 C O O H). Ammonium Hydroxide. 1: Bottle of Antacid tablets.B+ H2O ⇌ BH + + OH −.Base Strength of Acid: Ka: Name: Formula: Formula: Name: Strength of Base: Strongest: Large: Perchloric acid: HClO 4: ClO 4-Perchlorate ion: Weakest 3.
Strong and Weak Bases
Schlagwörter:IonsStrong and Weak Bases2 * 10 9: Hydroiodic . So if the vinegar is 3% acetic acid by mass and the molar mass of HC 2 H 3 O 2 = 60.1, it is likely a weak acid, which is a compound that is not 100% ionized in aqueous solution.1 for a strong acid HA can be represented with a single arrow: HA(aq) + H 2O(l) → H 3O + (aq) + A − (aq) Water is the base that reacts with the acid HA, A − is the . For example, if solid sodium hydroxide (NaOH) is placed in water, the solids will completely break apart into equal amounts of sodium ions (Na+) and hydroxide ions (OH-).
Classify the basicity of potassium hydroxide, KOH , based on its reactivity in aqueous solution.As it turns out, there are very few strong acids, which are given in Table 14.Schlagwörter:Thorough GuideIonsWeak BasesWeak Base Examples Hence, the ionization in Equation 16. Examples: NH 4 OH.By analogy, a strong base is a compound that is essentially 100% ionized in aqueous solution.Schlagwörter:Weak Base ExamplesExamples of Weak Bases Example HF and NH 3. Sodium bicarbonate. Aluminum hydroxide. Choose 1 answer: Weak .

Since there are no spectator ions, the total ionic and the net ionic are exactly the same. The term glacial refers to the fact that it would often solidify in many old, poorly heated chemical stockrooms.Geschätzte Lesezeit: 5 min Definition: A weak base is a base that is partially dissociated in an aqueous solution . A weak acid is an .HC2H3O2 ⇌ H + (aq) + C2H3O − 2 (aq) As it turns out, there are very few strong acids, which are given in Table 11. Examples for cations: Cation generated from a strong base: example Na + from NaOH NaOH(aq) . As Bronsted-Lowry bases are proton acceptors, a weak .Schlagwörter:Strong and Weak BasesStrong Acids and BasesStrong and Weak Acids Sodium hydroxide pellets: .April 17, 20249:07 AM PDTUpdated 28 min ago. A strong acid yields 100% (or very .1 includes some common strong acids.Schlagwörter:IonsStrong and Weak BasesStrong Acids and Bases
Weak
Combining a solution of many magnesium salts with basic water induces precipitation of solid Mg(OH)2 Mg ( OH) 2. Any acid that dissociates 100% into ions is called a strong acid.7: Strong and Weak Acids and Bases is shared under a license and was authored, remixed, and/or curated by LibreTexts. Kb = BH+ OH − B.001 M NH 3 (Table 1 from Ions in Solution) is slightly above 10 percent that of 0. The higher hydroxide means a lower hydrogen concentration, therefore, a greater pH.As will be evident throughout the remainder of this chapter, there are many .By choosing names that mean weak, parents can promote a more inclusive and diverse understanding of strength, encouraging their children to embrace their authentic selves.Only cations that are the conjugate acid of a weak base are strong enough to undergo hydrolysis. While Arrhenius bases are used as aqueous solutions, the superbases deprotonate water, reacting with it completely.Superbases are Lewis bases that are Group 1 salts of carbanions, such as hydrides and amides. Acetone: C 3 H 6 O. Assume that the vinegar is really just a solution of acetic acid in water, and that density = 1 g/mL.As it turns out, there are very few strong acids, which are given in Table 17. If the ionization reaction is essentially complete, the acid or base is termed strong; if relatively little ionization occurs, the acid or base is weak. Two weak bases: 1.
- Wc3 Reforged , Easy Guide: Warcraft 3 Reforged Customkeys Download (2020)
- Wayne Fontana And Mindbenders _ Wayne Fontana: 1960s pop star dies at 74
- Weber Brennersatz Spirit 320 , Weber Brenner Spirit E 310/320 ab 2013
- Watts To Dbm Calculator | dB converter
- Webcam Leasow | Webcams around Amlwch
- Wayss Freytag Ingenieurbau Ag Hamburg
- Ways To Eat Natto , 11 Easy Japanese Natto Recipes For The Beginner
- Web De Handy Tarif : Mobilfunktarife
- Wc Abflussspirale – Rohrreinigungsspirale: So wendet ihr eine Abflussspirale richtig an
- Watson Iot Hauptstadt München _ IBM Watson IoT Hauptsitz in München eröffnet
- Web Map Service , Web Map Service
- Wattwürmer Salz Einplanen , Schüsslersalze Anwendungsgebiete: Würmer
- Webex App Kostenlos Herunterladen