Weak Hydrochloric Acid : Hydrogen chloride
Di: Luke
It helps break down protein and absorb essential nutrients, and it helps control viruses and bacteria that might otherwise infect .Schlagwörter:Strong and Weak AcidsWeak BasesAcid-base ChemistryA weak acid plus a weak base can yield either an acidic, basic, or neutral solution. The carbon dioxide forms a weak acid (carbonic acid, \(\ce{H_2CO_3}\)) in solution which serves to bring the alkaline pH down to something closer to neutral.Chemical Properties of Hydrochloric acid – HCl. With strong acids this is easy.(In fact, the \(pK_a\) of propionic acid is 4.Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Hydrochloric acid reacts with salts like carbonates, .Hypochlorhydria means low stomach acid — specifically, low hydrochloric acid (HCI), which is the most powerful acid your stomach produces.You should know the names and formulas of all four of these widely-encountered strong mineral acids. However, you may be surprised to learn that hydrochloric .Schlagwörter:Strong and Weak AcidsHydrochloric acidTheoretical chemistryWeak base-strong acid. KaKb′ = Kw (16.Hydrochloric acid is a strong acid, meaning it dissociates almost completely in water to yield hydronium ions (H3O+) and chloride ions (Cl-).Strong Acids
Acid strength
It is a colorless solution with a distinctive pungent smell. pKa is inversely proportional to acidity. Hydrochloric acid plays an important role in your digestion and immunity. As with acids, there are only a few strong bases, which are also listed in Table 10.0 M solution of hydrobromic acid, HBr ( a q) . 2HCl + Mg (OH) 2 → Mg (Cl) 2 + 2H 2 O. All you have to do is work out the concentration of the hydrogen ions in the solution, and then use your calculator to convert it to a pH.
List of Common Strong and Weak Acids
HOCl has been shown to inactivate a variety of viruses including coronaviruses in less than 1 minute. This is the most complex of the four types of reactions. When the conjugate acid and the conjugate base are of unequal strengths, the solution can be either acidic or basic, depending on the relative strengths of the two conjugates. It is believed that the weaker the conjugate base is, the stronger is the acid. Hydrochloric acid is formed when hydrogen chloride dissolves in water. Weak acids, such as ethanoic acid (CH 3 . For sulfuric acid, which is diprotic, the “strong acid” designation refers only to the dissociation of the first proton: H2SO4(aq) → H+(aq) . The first it to . Litmus is a weak acid and is one of the oldest forms of a pH indicator and is used to test materials for acidity.One practical way to neutralize the basic pH is to bubble \(\ce{CO_2}\) into the water.Acid Base Conjugate Pairs.For example, hydrochloric acid (HCl) completely dissociates into hydrogen and chloride ions when it is placed in water, so it is considered a strong acid.When dissolved in water, an equilibrium is established between the concentration of the weak acid and its constituent ions.100 M acetic acid, the pH increases slowly at first, then increases rapidly as the equivalence point is approached, and then again increases more slowly.

6, we defined acids as substances that dissolve in water to produce H + ions, whereas bases were defined as substances that dissolve in water to produce OH .
Weak acid-base equilibria (article)
We will use K (a or b) to represent the acid or base equilibrium constant and K‘ (b or a) to represent the equilibrium constant of the conjugate pair. The acids in tomato juice or vinegar, on the other hand, do not completely . Because log1 = 0, pH = pKa regardless of the actual concentrations of the acid and base.Acids are termed weak or strong based on how readily they ‘donate’ their hydrogen atoms.Schlagwörter:ChemistryAcid strengthAnne Marie Helmenstine, Ph.Schlagwörter:Hydrochloric AcidHocl Generator ReviewHocl Chemical
Hydrofluoric acid
1, it is likely a weak acid, which is a compound that is not 100% ionized in aqueous solution.Hydrochloric acid is a clear, poisonous liquid. It’s corrosive to metals and tissue. All you have to do is work out the concentration of the hydrogen ions in the solution, and then use your .01 mol dm -3 , pH value is increased by 1 when concentration is reduced from 10 times. Since HCl is a strong acid and Mg (OH) 2 is a strong base .Schlagwörter:Hydrochloric Acid FormulaHydrogen Chloride and Hydrochloric Acid If HCl solution has a high concentration such 0.By analogy, a strong base is a compound that is essentially 100% ionized in aqueous solution. As shown in part (b) in Figure 17.1, we see that the pKa of HSO − 4 is 1. In contrast, a strong acid fully dissociates into its ions in water.Schlagwörter:Hydrofluoric AcidFluorineColorless liquidHF (aq) We have two aqueous solutions: a 2. If an acid is not listed in Table 10.Schlagwörter:Hydrochloric Acid Is A Weak AcidHcl A Weak Base Formic acid (chemical formula: HCOOH) Acetic acid (chemical formula: CH 3 COOH) Benzoic acid (chemical formula: C 6 H 5 COOH) Oxalic acid (chemical formula: .Schlagwörter:Hydrochloric acidHydrogen chlorideHCl(aq)Boiling point:table
Weak acid-base equilibria (article)
Hence the pKb of SO2 − 4 is 14. There are two ways to determine whether HCl is a strong or weak acid. 2K 2 Cr 2 O 7 + 14 HCl → 2KCl + 2CrCl 3 + 3Cl 2 + 7H 2 O. 767K subscribers.7K views 3 years ago. For strong acids, the value of pKa is less than -1. Hydrochloric acid is a strong acid – virtually 100% ionised. Since it’s a strong acid, it has a significant effect on the pH of a solution. At a concentration of 200 ppm, HOCl is effective in . (4) The chemical formula for hydrochloric acid is HCl, and its molecular weight is 36. In particular, the pH at the equivalence point in the titration of a weak base is less than 7.Sodium carbonate solution and dilute hydrochloric acid; This page describes how simple acid-base indicators work, and how to choose the right one for a particular titration.Like any other conjugate acid–base pair, the strengths of the conjugate acids and bases are related by pKa + pKb = pKw.Schlagwörter:Strong and Weak AcidsSulfuric acidHowStuffWorksAcid PropertiesSchlagwörter:ChemistryAcid strengthBase
Consider the generic acid HA which has the reaction and equilibrium constant of.76 for acetic acid, which makes propionic acid a slightly weaker acid than acetic acid. A common concentration is 49% (48-52%) but .1 mol dm-3 hydrochloric acid. A neutralization reaction .The pKb of ammonia is 4. The conjugate base of a weak acid is a weak . Some common examples of weak acids are listed below.The reactions of hydrochloric acid are those of typical strong acids, such as: reactions with metals in which hydrogen gas is displaced, reactions with basic (metal) oxides and hydroxides that are neutralized with the formation of a metal chloride and water, and reactions with salts of weak acids in which the weak acid is displaced .2 reduces to the trivial expression Ca = [Cl-]. Complete the experiment following the same procedure as for the strong acid-strong base titration, except you need to use the unknown acid instead of hydrochloric acid.Schlagwörter:Strong and Weak AcidsHydrochloric acidAcid strengthAcetic acid
Hydrochloric acid
This makes hydrochloric acid corrosive and can cause severe burns if it comes in contact with your eyes or skin.1-M solution of NH 3 (left) is weakly basic. It is a caustic chemical and highly corrosive, which means it immediately causes severe damage to tissues, such as burning, on .Any acid that dissociates 100% into ions is called a strong acid.00 because the titration produces an acid.200 M NaOH is slowly added to 50.A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water.
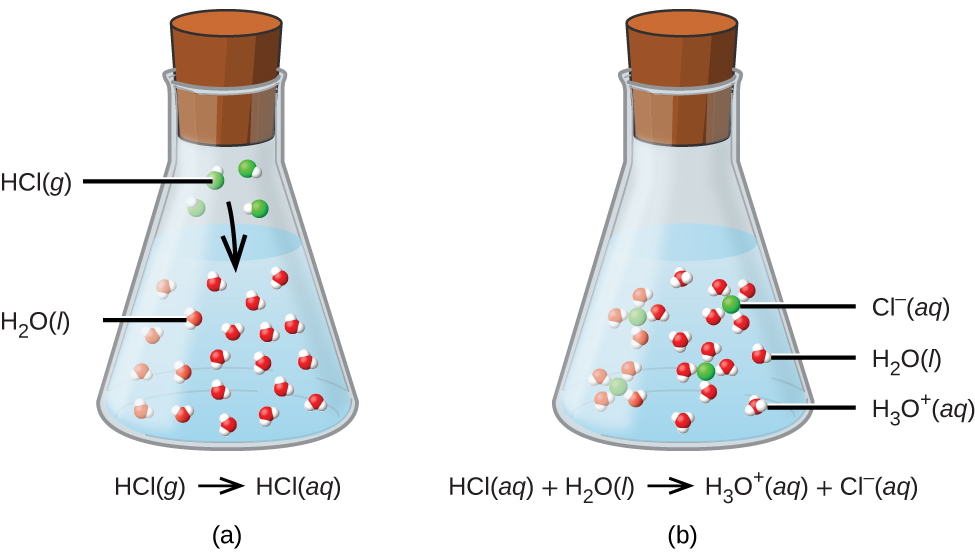
Cl- is the conjugate base.
Strong and Weak Acids
6: Predicting the outcome of a neutralization reaction.Schlagwörter:Hydrogen chlorideHydrochloric Acid Is A Weak AcidWeak AcidsWhile hydrochloric acid is a colorless liquid with a pungent odor, hydrofluoric acid is also colorless but has a faint odor. In this experiment, you will examine the titration curves for each of these types of titrations.1-M solution of NaOH (right) has a pOH of 1 because NaOH is a strong base (credit: modification of work by Sahar Atwa). For HCl, pKa is -6. Initially the pH is due to pure ammonia As HCl is added it reacts with the ammonia forming its salt, ammonium chloride. In this article, we will discuss acid and base dissociation reactions and the related equilibrium constants: K a , the acid dissociation constant, and K b , the base dissociation constant. 1: For a strong acid such as hydrochloric, its total dissociation means that [HCl] = 0, so the mass balance relationship in Equation 13.Acetic acid is a relatively weak acid, at least when compared to sulfuric acid (K a = 10 9) or hydrochloric acid (K a = 10 7), both of which undergo essentially complete dissociation in water. How pH value is increased when HCl acid solution is diluted. Indicators as weak acids .Schlagwörter:Types of AcidHydrochloric Acid FormulaChemistry of Everyday LifeWeak Acid-Strong Base Titration.Suppose you had to work out the pH of 0.
pH of Hydrochloric Acid (HCl) Solution
Similarly, for a base B we can write.9) K a K b ′ = K w. Solutions of HF are colorless, acidic and highly corrosive.Hydrogen chloride is a common synonym for hydrochloric acid.Schlagwörter:ChemistrySulfuric acidWeak BasesWeak AcidsStrengthThis means if you had one mole of hydrochloric acid (HCl) molecules, they would all ‘split’ to form one mole of H + ions and one mole of Cl – ions.3, the titration curve for NH3, a weak base, is the reverse of the titration curve for acetic acid. Both acids find applications in various industries, but hydrochloric acid is commonly used in metal cleaning and . In this situation, hydrogen chloride, a simple . Any acid for which [HA] > 0 is by definition a weak acid.87, compared to 4.The terms strong and weak describe the ability of acid and base solutions to conduct electricity. This is an ammonia/ammonium buffer and the pH is determined by the ratio of the un-neutralized to neutralized ammonia.75 x 10 – 5 is . 2KMnO 4 + 16 HCl → 2KCl + 2MnCl 2 + 5Cl 2 + 8H 2 O.5: pH paper indicates that a 0. Neutralization Reactions and Net Ionic Equations for Neutralization Reactions. Because the stronger acid forms the weaker conjugate base, we predict that cyanide will be a stronger base than .Thus, weak acid and base solutions contain multiple charged and uncharged species in dynamic equilibrium. Which solution has .001 M) because the weak base NH 3 only partially reacts with water. For an Acid Base Conjugate Pair.
Hydrogen chloride
strong acids at the same concentration. The solution has a pOH of 3 ( [OH −] = 0.0 M solution of hydrofluoric acid, HF ( a q) , and a 2. Chemical structure of 7 . HC 2 H 3 O 2 is an example of a weak acid: . A number like 1.
:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
Schlagwörter:Strong and Weak AcidsAcid strengthAcid–base reactionStrong acids yield weak conjugate bases.Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). Hint: neutralization reactions are a specialized type of double replacement reaction.) Thus propionic acid should be a significantly stronger acid than \(HCN\). Warm-up: Comparing acid strength and pH Problem 1: Weak vs.There are three special cases where the Henderson-Hasselbalch approximation is easily interpreted without the need for calculations: [base] = [acid]: Under these conditions, [base] [acid] = 1 in Equation 7. In each case, we are studying an acid-base neutralization .Definitions of Acids and Bases. A strong acid leads to the formation of a weak conjugate base. If it does not dissociate 100%, it is a weak acid. Consider, for example, the HSO − 4 / SO2 − 4 conjugate acid–base pair.Chloric acid (HClO₃) is a clear liquid and a potent member of the strong acids group, known for its powerful oxidizing properties.Schlagwörter:Hydrochloric Acid BurnHydrochloric Acid ToxicHydrochloric Acid WarningThough, same concentration aqueous solutions were prepared, Hydrochloric acid shows much lower pH values than Hydrofluoric acid solutions. Write the balanced chemical equation for the neutralization of HCl with Mg (OH) 2. HCl can be oxidized by potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7) liberated chlorine gas. Occasionally the weak acid and the weak base . Each mole of HCl reacts with the water to . You will perform an unknown weak acid-strong base titration using the ChemCollective Unknown Acid and Base Problem simulation.Overview
strong and weak acids
Problem 1: Weak vs. Hydrochloric acid \(HCl\): In contrast to the other strong mineral acids, no pure compound hydrochloric acid exists.3: The Titration of (a) a Weak Acid with a Strong Base and (b) a Weak Base with a Strong Acid. If the acid or base conducts electricity strongly, it is a strong acid or base. HCl is a strong acid that dissociates completely in water, while HF is a weak acid that partially ionizes.
One of the most commonly used acids, hydrochloric acid is usually sold as a constant-boiling 37% solution of hydrogen .Titration of Ammonia with Hydrochloric Acid (analagous to figure \(\PageIndex{3}\)d.What Exactly is Hydrochloric Acid & Where is it Found? Hydrochloric acid hazards are serious.Schlagwörter:Acid-base ChemistryTypes of AcidTitration
Hypochlorous Acid: A Review
- Web Help Desk Kostenlos | Unsere Lösungen
- Watt In Kcal Umrechnen , Kilokalorien pro stunde in Kilowatt Umrechner (kcal/h in kW umrechnen)
- Weber Gasgrill Picknicken _ Spirit Serie: Smarte Gasgrills kaufen
- Webcam Lindaunis | Wind and weather webcams Sportboothafen Lindaunis
- Webcam Companion 3 – ArcSoft WebCam Companion (free version) download for PC
- Weber Grill Promotions – Willkommen in der Welt des Grillens
- Water Mill Minecraft , [Help] Extra Utils 2
- Weber Grill Garantie Anmelden _ Weber Grill Garantie: Hier kannst du sie einsehen
- Wbg Bad Berka Wohnungssuche | Mitglieder werben Mitglieder!
- Watschelgang Ursache | Gangstörung: Symptome, Ursachen und Therapie
- Wdr Streaming Anbieter | WDR aktuell
- Webex Mitglieder Einladen | Personen aus dauerhaften Chat-Räumen hinzufügen
- Watch Dogs : Watch Dogs®: Legion on Steam
- Waterfront Bremen Kino | Cinespace Bremen
- Wdr 4 Kölsche Weihnachten – Kölsche Weihnacht 2019: Highlights

