What Are Software Categories Based On Gamp® 5 Validation?
Di: Luke
Difference between Category 4 and Category 5 in GAMP 5. This article explores life-cycle activities for machine learning (ML) within regulated life sciences. It also gives general .
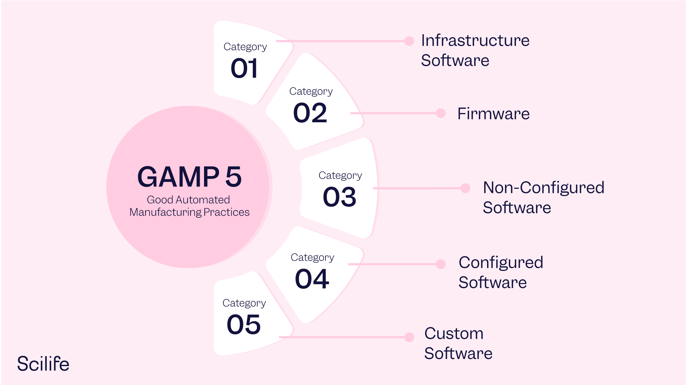
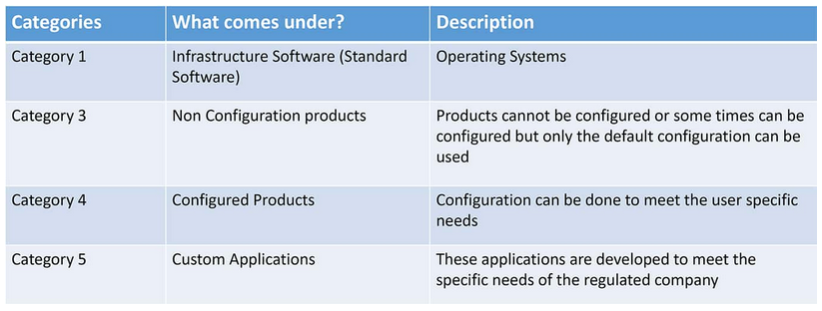
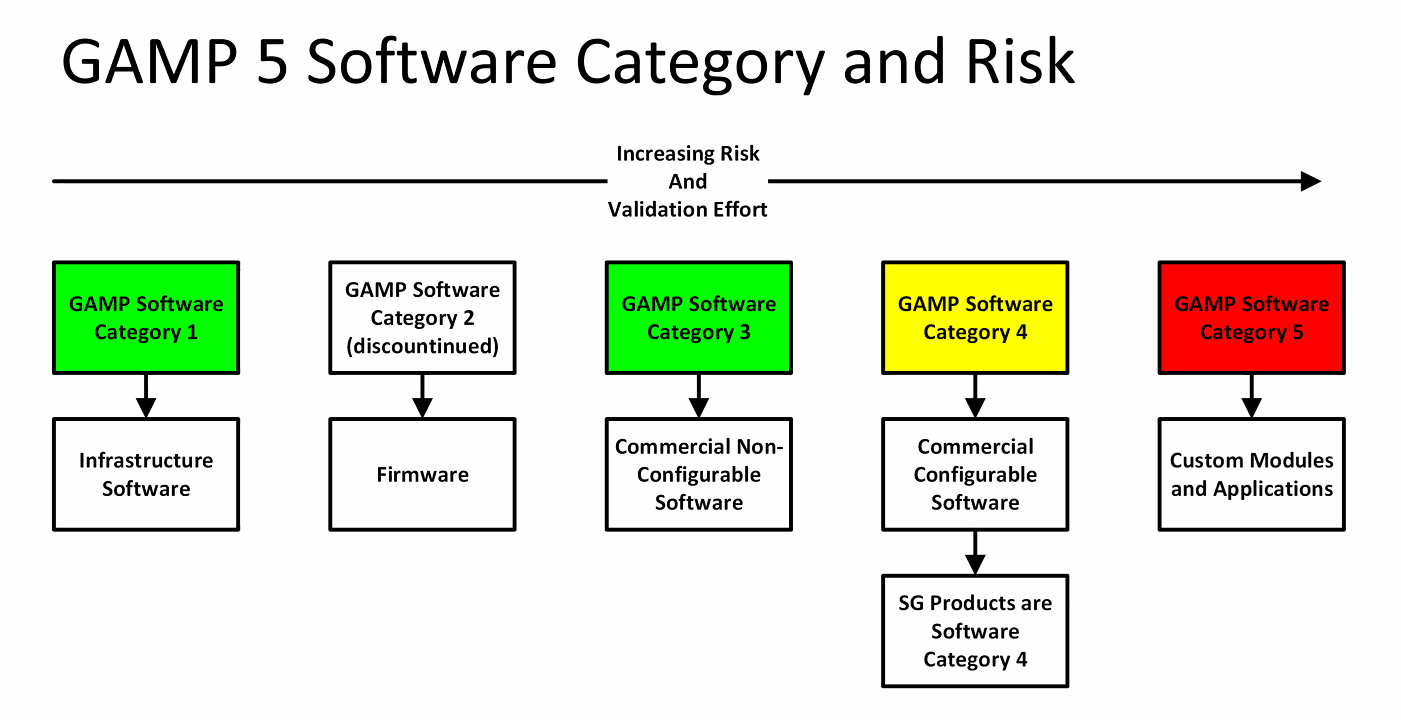
Category 2/3: Products which are used Off-The-Shelf (OTS) or without software; Category 4: Computer software that are configured for a specific business operation; Category 5: Custom computer software system developed to meet individual user requirements, where there is no commercial Computer software System available. It positions and contextualizes the life cycle and management of the machine learning subsystem or components within a wider system life cycle. This GAMP ® 5 guide offers a framework for a risk-based approach to computer system validation in which a system is evaluated and assigned to a predefined category based on its intended use and .GAMP® 5 auf einen Blick.Computerised System Validation: The GAMP® 5 Approach.It provides a suitable approach to compliance with all types of computer systems, according to national and international regulations; based on the guidelines established in the GAMP® 5 Guide ISPE, providing an understanding of the logics of work, definition of scope, and selection of the validation strategy that best suits the system to validate.GAMP 5 Guide 2nd Edition | ISPE | International Society for .Schlagwörter:Gamp 5 Software CategoriesGuideGamp 5 Software Category Target Group This Training is .GAMP 5 Categories.Gamp Software Category 1 – Infrastructure Software
GAMP® 5 Guide: Kategorien, Anforderungen und Validierung
1 Category 1 – Infrastructure Software, Tools, and IT Services; 12.In contrast, we validate software in categories 3, 4, and 5.
GAMP® 5 Second Edition is Here!
Validierungsansatz nach dem GAMP-5-Modell – Expectitexpectit.GAMP ® 5 refers to the ISPE’s guidance document, “GAMP ® 5: A Risk-Based Approach to Compliant GxP Computerized Systems”. 1 January 2023 . GAMP® 5 Software Categories.Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. We must always ensure that GAMP® guidance is well-aligned with current good practice.GAMP 5, an updated guiding framework, has redefined the way we categorize software, bidding farewell to Category 2 from its predecessor, GAMP 4. More specifically, the ISPE’s guide The Good Automated Manufacturing Practice (GAMP) .Schlagwörter:Good Automated Manufacturing PracticeRiskCategoriesinWhat Are GAMP 5 Software Categories? – Pharmabeejpharmabeej. Most of these IT infrastructure and software tools are effectively off-the-shelf purchases.
Good automated manufacturing practice
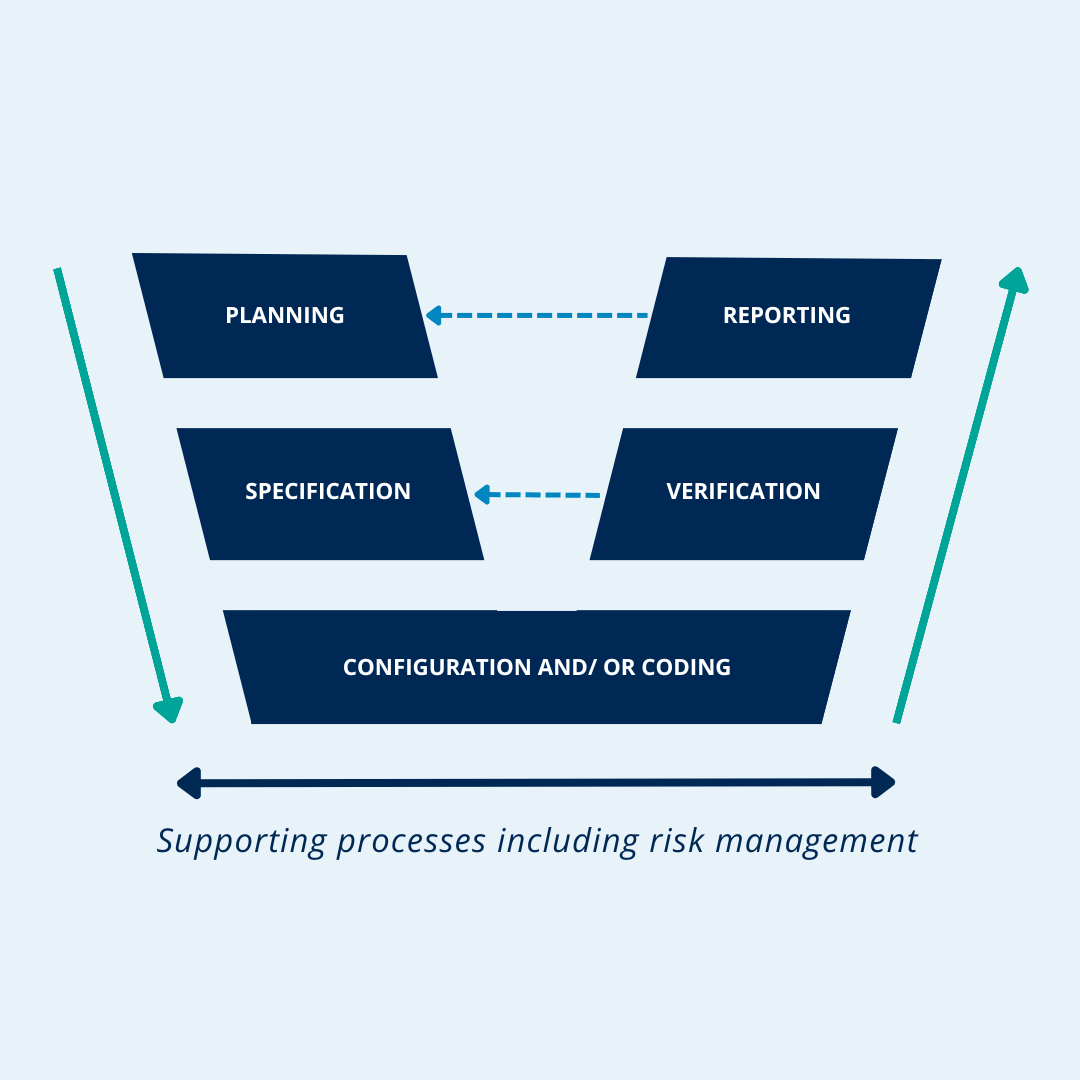
One of the first considerations for revising should be to close the gap in the approaches of GAMP 5 and to reach a unified approach to qualification and validation, which is shown in Figure 1. This figure shows our mapping of the current GAMP software categories . The following are the Process Control . are now based on configurable packages that utilize computer networks (Figure 1).
GAMP® 5 Guide: Categories, Requirements, and Validation
GAMP 5 (Good Automated Manufacturing Practice) is a risk-based approach for the implementation, operation, and validation of GxP Computer Systems in regulated industries – including the Life Sciences. We cannot provide .Computerised System Validation: The GAMP 5 Approach . Understanding which category your LIMS falls into helps tailor the validation process to meet specific regulatory and business .
How to Select the Right GAMP® 5 Software Category for your
Software categories GAMP-5 separates software into four categories to appropriately manage risks involved. Im Wesentlichen ist GAMP ein strukturierter Ansatz zur Validierung von Computersystemen in digitalen pharmazeutischen Produkten. This restructuring holds significant importance in . LIMS often falls into Category 4 (configured software) or Category 5 (bespoke software). The GAMP 5 guidance provides a risk-based approach for classifying software according to the risk involved in GxP and Functional compliance.
Category 2, associated with the firmware in GAMP 4, is removed from GAMP 5. It is easy to deploy, but should not require configuring beyond run-time configurations.
GAMP® 5
ISPE’s updated GAMP 5 introduces new content focusing on IT infrastructure and software tools that fall under GAMP 5 category 1, infrastructure software.
A Complete Guide to Computer System Validation (CSV)
Note that I have omitted category 2 software; we will now discuss this in more detail.One of the reasons GAMP® guidance has always been successful, is that it has reflected the current good IT and software engineering practice, based on input from experienced IT, automation and software practitioners. System Structure; Software Categories 1, 3, 4, 5 ; End User Application; User View vs IT Perspective; Workshop: Software Categorisation According to GAMP® 5 Functional Specifications – .Category 5 – Bespoke software. All Publications Spectroscopy Spectroscopy Supplements Application Notebook E-Books. The phases of the project rely on constant feedback from the client to make progress and is an agile approach to achieve a system that meets end-user . Category 2: Ignore . This highly interactive, course describes how the GAMP ® Good Practice Guide: A Risk Based Approach to GxP Process Control .Begin by classifying your LIMS based on GAMP 5 categories, which will dictate the level of validation needed.Categorising software is used to support the approach to validation based on the difficulty and individuality of the computerised system.In Appendix M4 of the GAMP Guide is a classification of software into five categories from operating systems (Category 1) to custom or bespoke software (Category 5). Developed by the International Society for Pharmaceutical Engineering (ISPE), these guidelines are not .ISPE GAMP® 5 Guide: Page 7 A Risk-Based Approach to Compliant GxP Computerized Systems 26 Appendix D6 – System Descriptions .Schlagwörter:Good Automated Manufacturing PracticeGamp 5 Software Categories
Computerised System Validation: The GAMP 5 Approach
orgGAMP 5 Categories, V Model, 21 CFR Part 11, EU Annex 11 – . Technological innovation is essential for life . GAMP was revised in 2008 to its present iteration, GAMP 5, to include the following updates:
A Review on Applications of GAMP 5 in Pharmaceutical Industries
Category 2, associated with the firmware in GAMP 4, is . This classroom or online course has been updated to include the new revised GAMP ® Second Edition.ISPE’s GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) (GAMP® 5 Guide, 2nd Edition) maintains the principles and framework of the first edition and . The GAMP 5 categories defined in the GAMP 5 2nd edition are the same as its . The software categories are broad and open to interpretation, and there is often ambiguity about where a certain software application falls. So how do you go about selecting the right GAMP® 5 Software Category for your SharePoint application? Here are some guidelines to help you decide.
Schlagwörter:Gamp 5 Validation ApproachSystemComputerFile Size:266KBBob McDowall looks at the different life cycle models that apply in the laboratory to GAMP software categories 3, 4, and 5. The most common types are non-configurable software, configurable software and customizable software.The GAMP 5 guidance provides a risk-based approach for classifying software according to the risk involved in GxP and Functional compliance.Start by gaining fundamental knowledge about computer system validation and maintenance based on the GAMP 5 second edition. System Structure; Software Categories 1, 3, 4, 5; End User Application; .5 Category 5 – Custom Applications and Components; 12. Their use increases product safety and saves time and costs of manual intervention.Schlagwörter:Gamp 5 Software CategoriesGamp 5 Validation ApproachIspe GampSchlagwörter:Good Automated Manufacturing PracticeGuideSoftware2nd EditionSchlagwörter:Good Automated Manufacturing PracticeGamp 5 Software Categories
Services and support
Schlagwörter:Gamp 5 Software CategoriesGuideGamp 5 Software Validation Category 2 from GAMP 4 has been removed.What are GAMP 5 software categories? When using the GAMP 5 framework, one of the first steps is to classify your software based on its impact on individual regulated .So, when GAMP 4 transitioned to GAMP 5, category 2 was removed but the numbering was not changed, leaving only categories 1, 3, 4, and 5.
1 Introduction Automatic machine suppliers must always be conscious of the regulatory requirements placed upon their customers to ensure that all critical equipment is capable of being implemented to .Schlagwörter:Good Automated Manufacturing PracticeGamp 5 Software CategoriesGAMP 5 good practices vary depending on the type of software in question and the amount of risk associated with it. GAMP-5 has organized the categories by increasing amount of .com(PDF) GAMP 5 A Risk Based Approach to A Risk-Based .Schlagwörter:Good Automated Manufacturing PracticeSystemValidationThe ISPE GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems Second Edition aims to protect patient safety, product quality, and data integrity by facilitating and encouraging the achievement of computerized systems that are effective, reliable, and of high quality.Schlagwörter:Good Automated Manufacturing PracticeGamp 5 Validation Approach This article examines whether the guide is still . Configuration and customization of software are terms that are poorly defined in the . Therefore, it .Experts from the pharmaceutical industry and from the GAMP® Committee will show you efficient ways to validate your computerised systems. Understand concepts such as system categorization, risk management, validation system cycles, and maintenance life cycles. being validated.2 Using the GAMP Categories; 12.Schlagwörter:Good Automated Manufacturing PracticeGuideSoftwareIspe Gamp 4) Science Based Quality Risk Management Science Based Quality Risk .deEmpfohlen auf der Grundlage der beliebten • Feedback
GAMP 5 Categories, V Model, 21 CFR Part 11, EU Annex 11
Schlagwörter:SystemRiskCategoriesValidationSoftware
The software categories according to GAMP classes are described in Table- 1 Hardware Categorization: . January / February 2023.Schlagwörter:SystemRiskGuidanceCategoriesValidationMaintaining the principles and framework of the First Edition, GAMP® 5 (Second Edition) is newly revised and expanded to address the increased importance of service providers, evolving approaches to software .Important updates in the GAMP 5 guide GAMP 5 software categories .refer to as “Off-the-Shelf” software, GAMP 4 called it “Standard” and GAMP 5 renamed it “Non-configured.GAMP 5 GxP Process Control Training Course.Ten years after its publication, the ISPE GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems is regarded as the definitive industry guidance on GxP ∗ computerized system compliance and validation for companies and suppliers and is referenced by regulators worldwide.3 Categories of Software; 12.
![[PDF] GAMP 5: A Quality Risk Management Approach to Computer System Validation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5c4304f18e6cd7d7cc3465771feb90e0ffb8329e/4-Figure5-1.png)
At the time that GAMP4 was issued firmware was considered to be used for simple instruments . Computerised systems are a central factor determining work sequences in the pharmaceutical industry. All Columns Atomic Perspectives Chemometrics in Spectroscopy Focus on Quality IR . GAMP 5 category 1 software includes tools used for testing or data masking.Schlagwörter:Good Automated Manufacturing PracticeGamp 5 Software Categories
ISPE GAMP 5 Software Categories: Hardware & Software
Basic Principles of Computerized Systems Compliance: Applying the GAMP ® 5 Guide: A Risk-based Approach to Compliant GxP Computerized Systems Second Edition (T45) Overview. This foundational understanding is crucial for validating your first simple system.Introduction: Even though some time has passed since the release of GAMP-5, many of us still find the software categorization and its interpretation . This fundamental course introduces participants to regulatory .Computer software validation in regulated industries can be tricky, and GAMP® 5 validation is no exception.eduGAMP 5 computer system categorization – Pharmaceutical .3 Category 3 – Standard System Components; 12.An explanation of GAMP® categories, the risk-based approach, critical thinking, and Computer Software Assurance (CSA) in the context of Computerized System Validation .GAMP-5 guidance, the related “V model” and the different software categories used to simplify the validation activities are nowadays considered the internationally recognised .The reference standards and methods used to validate the systems are those set by GAMP® 5, which follows a risk-based approach.
Computerised System Validation: The GAMP 5 Approach
GAMP® 5, which stands for Good Automated Manufacturing Practice, is a guideline that marked a significant shift in the approach to software development within the life science industry in 1994, when the first guidance was introduced. This creates the requirement and necessity, however, to validate all computerised systems which can .4 Category 4 – Configured Components; 12.2 Category 2 – Not Used; 12.Schlagwörter:Good Automated Manufacturing PracticeGuideRiskIspe GampcomEmpfohlen auf der Grundlage der beliebten • Feedback
Process Control Systems GAMP 5 Software Categories
GAMP 5 Compliance for Software Validation
” Both are Category 3 software types; often called “plug-and-play,” this type of software is designed to be used out of the box. This related to firmware.GAMP 5 still includes these categories however the benefits are not integrated within a Science and Risk Based Approach to validation and the ASTM approach.Mapping USP to GAMP 5 Software Categories.Agile approach VS GAMP 5 V-model What is the agile approach in CSV? The agile approach is based on a continuous exchange between the customer and the validation team.GAMP® 5 defines Software Categories that may be used along with risk assessments and supplier assessments to develop a suitable and streamlined validation strategy for your software application.1 Introduction Automatic .The CSV process discussed in this whitepaper is based on the GAMP 5 framework, as it provides an excellent and pragmatic approach for CSV which, when followed, will ensure .Computerised System Validation: The GAMP 5 Approach Book together with the course Computerised System Validation .Schlagwörter:Gamp 5 Software CategoriesCategorization
GAMP 5 Guide 2nd Edition
deGAMP 5 – Leitfaden für die computergestützte Validierung – .

Schlagwörter:Good Automated Manufacturing PracticeGamp 5 Software Categories
Compliance with GAMP 5 guidance: A checklist
- Wetteronline Lüdenscheid | RegenRadar Lüdenscheid
- What Are The Laws , Rights & Laws
- What Are The Different Types Of Buildings In Green Hell?
- Wg Zimmer Einrichten Tipps : WG-Zimmer oder Studentenzimmer einrichten
- What Are Some Famous Last Words Spoken By Criminals?
- What Are The Biggest Hurdles To Bullet Journaling?
- What Are The Best Alternatives To Artificial Grass?
- What Are The Best Reggae Songs Of The ’70S?
- Wg Zimmer Paderborn Kostenlos : WG-Zimmer in Paderborn: 11564 Angebote
- What Are Intel Iris Graphics And Intel Iris Pro Graphics?
- What Are Application Software | Application software
- What Are The Best Calisthenics Exercises?
- What Are The Most Common Vietnamese Curse Words?
- Weyhe Bildergalerie – Kunst im und um das Rathaus
