What Is A Sacrificial Anode? – Sacrificial Anodes FAQs
Di: Luke
The inexpensive metal anodes possess low oxidation potential, which could prevent undesired overoxidation of substrates, active intermediates and products. The simplest systems consist of the selection of an anode fabricated from an active metal (normally, zinc, aluminum, or magnesium).
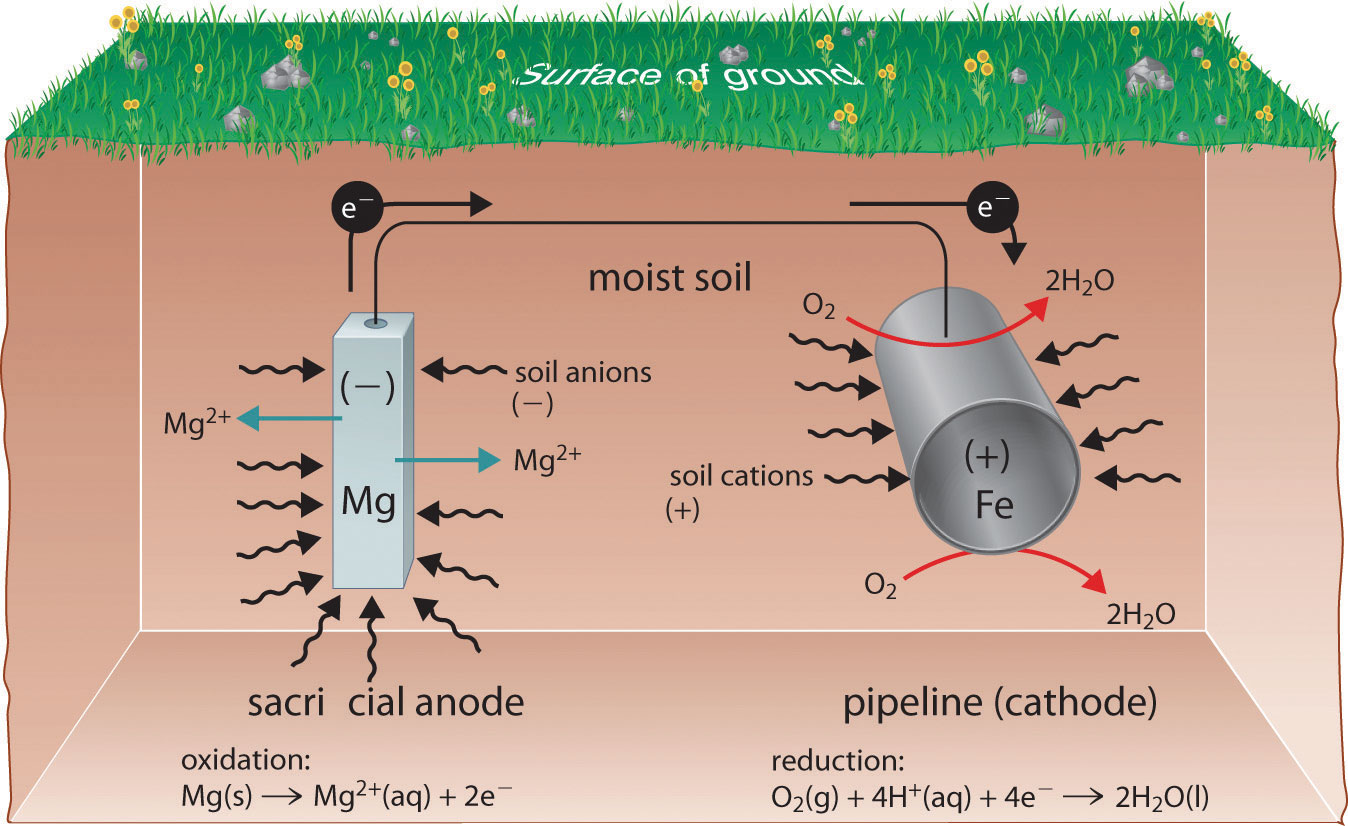
They rely on the natural potential differences between the two metals to drive the cathodic protection current. Sacrificial Anode: Venturing .Sacrificial Anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect.

MME Group: Sacrificial Anodes
Method #1: Sacrificial anode. Sacrificial Anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. These metals are more reactive . Water heaters have a sacrificial anode because, through an electrochemical mechanism, the sacrificial collects particles of iron, limestone, or . For instance, when protecting iron, you would use a metal that is more reactive than iron, such as zinc or magnesium.Sacrificial anode cathodic protection systems are also in some cases less costly to install and maintain than impressed current cathodic protection systems. Magnesium is the most active type of galvanic .A sacrificial anode system is a form of protecting submerged structures from CORROSION by using sacrificial anodes, also known as galvanic anodes, which are the . For one, they protect your water heater from corrosion by attracting damaging elements like minerals and sediment.The source can be a sacrificial anode of zinc or aluminum, or a line-operated or photovoltaic power supply.What is a galvanic or sacrificial anode? Galvanic anodes are basically metal castings that do not utilize an external power supply to drive current. It is called a sacrificial anode .Sacrificial Anodes are highly active metals that are used to prevent a less active material surface from corroding.The whole point of a sacrificial zinc anode is to actually set up electrical corrosion that favours the steel at the expense of the zinc.Sacrificial anodes, also known as galvanic anodes, are the protection mechanisms that you need to employ against corrosion. Without a sacrificial anode rod, your steel water tank . It is the piece of metal on the outside of the tank that gets corroded, and it’s important to have one because corrosion damages pipes.Sacrificial anode cathodic protection; Impressed current cathodic protection, often referred to as ICCP; You may also read about. While they don’t stop . Your geyser is an important part of the home and is also a costly item to replace. The in situ generated metal ions from sacrificial anodes could not only serve as Lewis acids to activate the reactants but also as a promoter or mediator. The anode is made from a metal alloy with a more active voltage (more negative electrochemical potential) than the metal .The most active metal (zinc for example) becomes the anode to the others and sacrifices itself by corroding (giving up metal) to protect the cathode – hence the term . Marine structures are predominantly made of steel.
Why Do Water Heaters Have A Sacrificial Anode?
During a reductive .What is a sacrificial anode? 1.Sacrificial anodes are metals or alloys attached to the hull that have a more anodic, i.What a Sacrificial Anode Is.Sacrificial anodes are available in many different shapes and sizes. In other words, these materials are easily corroded, so they can take the . It quite literally ‘sacrifices’ itself so as to prevent corrosion within your hot water system. These are called as sacrificial . Cathodic protection ( CP; / kæˈθɒdɪk / ⓘ) is a technique used to control the . Types of zinc anodes include: inline anode, anode weight, bolt-on type for rails, and zinc anode for pool lighting.
Sacrificial Anodes: A Guide To Marine Corrosion Protection
The anode is a part of a water heater that can be replaced.
Galvanic anode
The sacrificial metal must however be replaced periodically as it is . Types of sacrificial anodes. As we explained in a recent post about the Folsom Pinhole Water Leaks, when two dissimilar metals are connected within a plumbing system, one metal will .

The sacrificial anode will be consumed in place of the metal it is protecting, which is why it is referred to as a sacrificial anode.Unfortunately, a sacrificial anode rod is called that for a reason. The simplest method to apply cathodic protection is by connecting the metal to be protected with another more easily .
Definition and function. Since water heaters are vulnerable to corrosion, it’s important to inspect . As the anode rod is made of a softer metal (magnesium) than the water tank, it bears the .Hidden and forgotten, water heater’s the anode rod, also known as “the sacrificial helper,” offers up its body in exchange for your water heater tank.As the name suggests, a sacrificial anode rod is a long metal rod that hangs down from the top of the water heater. Sacrifical anode cathodic protecion. It is sacrificing itself to save the lining of the tank. An example of a sacrificial anode is a block of zinc that is attached to a steel plate.
Everything You Need to Know About Anode Rods
Mg is widely used as a sacrificial anode to provide cathodic protection of underground and undersea metallic structures, ships, submarines, bridges, decks, aircraft and ground transportation systems. How Sacrificial Anode Works? A sacrificial anode is a metal piece attached to structures like ship hulls or pipes to prevent them from rusting. When the anode rod has rusted away . It would be hard to overstate the . This is particularly true for systems with small current requirements (0.
Why Are Anode Rods Important?
Replacing the Anode Rod in a Caravan Hot Water System
Geschätzte Lesezeit: 2 min
Sacrificial Anodes
Sacrificial anodes serve as a source of electrical energy.Sacrificial Anodes.The loss (or sacrifice) of the anode material gives rise to the alternative name of sacrificial anode.Our anodes function economically and efficiently without any maintenance during the operating period. They also have to be installed the right way or the same bad result will happen Besides the Electrical Potential, the next factor most important in Sacrificial Anodes is the Current Capacity of . In fact, most boaters refer to sacrificial anodes simply as zincs. These anodes supply the . But they also help extend the life of your water heater by helping to keep it maintained.A sacrificial anode is a metal rod found in your geyser to help reduce any. Monitoring the level of cathodic protection.
Sacrificial Zinc Anode
A sacrificial anode is a metal rod or electrode that is made from a material that is more easily corroded than the metal it is protecting.The sacrificial anode anti-electrolosys device is relatively easy to install, affordable and available in numerous shapes and sizes depending on where you would like to install it in your pool. Galvanic cathodic protection relies on the potential difference between the sacrificial anode and the cathode, or the material being protected from corrosion.The sacrificial anode is an essential component of your water heater. The definition might be a little science heavy for the lay person, but basically when you put salt in your pool it becomes a big electrolytic bath as salt water conducts (transfers) electricity much better than fresh water.Anode rods are crucial to your water heater’s performance.
How does a sacrificial anode work
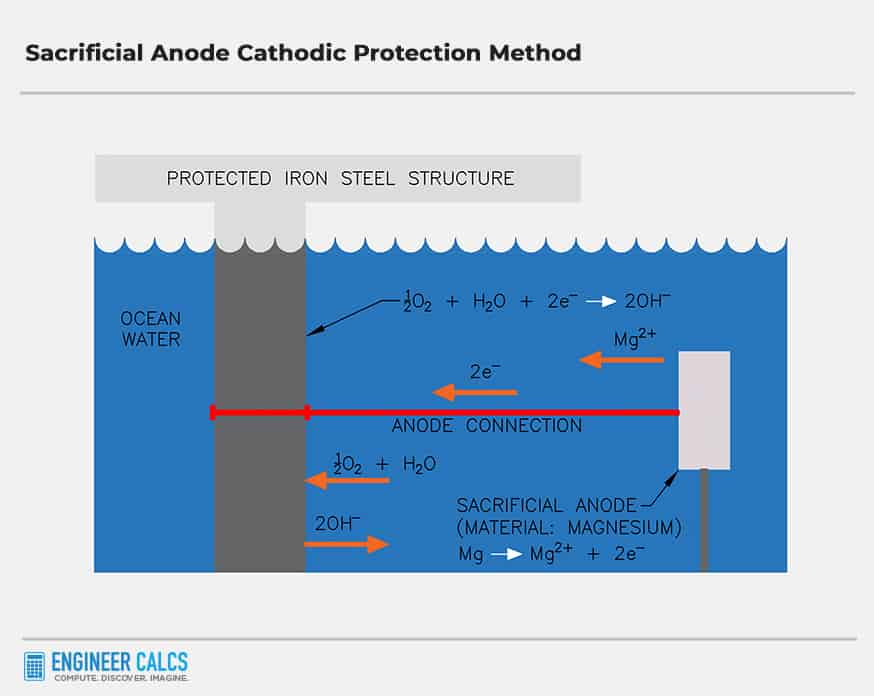
This piece of metal is called a sacrificial anode, and most often it is zinc.Knowledge: Sacrificial anodes Home What is sacrificial anode cathodic protection? A sacrificial anode system is a form of protecting submerged structures from corrosion by using sacrificial anodes, also known as galvanic anodes, which are the basis of traditional galvanic cathodic protection systems.Zinc anodes are active metals that prevent less active metals from corroding, making them a great way to protect metal equipment in saltwater pools. less noble, potential than steel when immersed in sea water. It can get confusing when it comes to pool chemistry but the principal behind a sacrificial anode is relatively simple. At some point, all of the magnesium or aluminum of the rod will have rusted away, and it will no longer have electrons to give up to save the tank’s electrons from the rusting process.
Sacrificial Anode
A sacrificial anode is a metal that is more reactive than the metal it is protecting. Caravan hot water system anode rod. This makes them highly . The strength and low resistance connections allow the anodes to be consumed in place of the structure, enabling cathodic protection to function . How does a sacrificial anode work? Lorem ipsum .Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (Equation \(\ref{Eq7}\)). A more reactive metal loses electrons more easily, forming ions.Sacrificial anodes are applied to the protected structure through a process involving welding or making mechanical connections with low resistance.A sacrificial anode system is a form of protecting submerged structures from corrosion by using sacrificial anodes, also known as galvanic anodes, which are the basis of . Image by Rémi Kaupp and used with permission.Magnesium is electrochemically the most active metal employed in common structural alloys of iron and aluminum. It’s a long metal rod, made of magnesium or aluminum, which extends through the tank’s interior. As other posters have pointed out, the throw of the zinc is limited when only damp and not in an electrolyte. High purity anodes (zinc, aluminum or magnesium) are required to avoid significant anode polarization and . Of these, the most important by far is electrochemical corrosion of metals, in which the oxidation process M → M + + e – is facilitated by the presence of a suitable electron acceptor, sometimes referred to in corrosion science as a depolarizer. Highly active meals are applied to a metal surface to protect that structure from corroding. The limited protection area is worked around by painting whole areas to be protected with zinc.
How to Choose a Sacrificial Anode
It “sacrifices” itself to protect the metal of your water heater tank. When the anode is connected to the metal object, it attracts corrosion away from the object, sacrificing itself in the process and extending the lifespan of the protected metal. Following the same principle of . Then that sacrificial anode is directly connected to the structure exposing .5 A or less per 100 lineal feet of structure). How sacrificial anodes work.Sacrificial anode cathodic protection (SACP) is a type of cathodic protection where a less noble material that acts as a sacrificial anode is connected by metallic .Sacrificial Anodes: Metal strips of top-order metals in the reactivity series serve as anodes and are installed for cathode protection.The Sacrificial Anode Rod is a part installed in your caravan hot water system, which does just as its name suggests.

There are three primary types of galvanic anodes.Table of Contents. What’s more, as a sacrificial anode supplier we are able to supply these anodes for ships in conjunction with Impressed Current Cathodic Protection (ICCP) systems.
Sacrificial Anode Cathodic Protection
The anodes in sacrificial anode cathodic protection systems need to be inspected periodically and must be replaced when consumed.Sacrificial Anodes have a working life to them and will wear down and eventually be of no use leaving the boat owner at risk for damage to their boat. Galvanic anodes (sacrificial anodes) must be periodically . There are no power costs or costs associated with furnishing power at a remote site associated .In simple terms, sacrificial anodes are blocks or rods made from highly active metals such as zinc, aluminum, or magnesium.Zinc sacrificial anode (rounded object) screwed to the underside of the hull of a small boat.Overview
Sacrificial Anode
Sacrificial Anodes FAQs
It’s also important to insulate the connections. The sacrificial anode, also known as the galvanic . For this reason, they do not lead to additional labor costs post-installation. The larger the potential is .What Does Sacrificial Anode Mean? Sacrificial anodes are easily corroded materials deliberately installed in a pipe or tank to be sacrificed to corrosion, leaving . Another effective way of protecting your saltwater pool equipment is . This tutorial review highlights .

What Is a Sacrificial Anode
The sacrificial anode will be consumed in place of the metal it is protecting, which is why it is . Zinc sacrificial anode (rounded object screwed to underside of hull) used to prevent corrosion on the screw in a boat via cathodic protection.As the name implies, a sacrificial anode is a material that experts install in pipes or tanks to make a sacrifice to corrosion.
What is a sacrificial anode?
One type of cathodic protection system is the sacrificial anode. The iron becomes a cathode which does not corrode, while the anode corrodes to provide the desired sacrificial protection.A typical sacrificial protection is where a more reactive metal is used as a sacrificial anode by attaching it to steel pipes or pump bodies. 1 Sacrificial metal anodes enable reductive electrosynthesis by charge-balancing the target reductive reactions at the cathode. The standard method for corrosion prevention.
- What Is A Family Changeover? – Gender pay gap remained stable over past 20 years in US
- What Is Average Total Cost? | Cost of a data breach 2023
- What Is A Special Case Of Amputation?
- What Is A Profibus Module? _ Profinet
- What Is An Oligarchy | Oligarchy
- What Is A Valet De Chambre? : Valet / Femme de chambre : métier, formation, salaire
- What Is A Molding Process? : Guide to Mold Manufacturing & Its Processes
- What Is Berlin Located In : Berlin Map
- What Is An Example Of A Pro Rata Discount?
- What Is A “Conservative” Interpretation