What Is An Example Of A Redox Reaction?
Di: Luke
So that’s just a mnemonic. A redox reaction is one in which both oxidation and reduction take place. In which, the atoms or the compounds gain and lose electrons simultaneously at the same time. Sometimes a half reaction must have all of its coefficients multiplied by some integer for all the electrons to cancel. Chemical reactions in which electrons are transferred are called oxidation-reduction, or redox, reactions. Reaction of Iron and Hydrogen Peroxide. In this tutorial, you will learn what a redox reaction is, the different parts of such a reaction, as well as how to recognize and write redox reactions. Learning Objectives.
Redox reaction
A line from the oxidizing agent Fe 3 + to the reducing agent Cu is downhill, and so the reaction will occur. Some everyday examples . This redox reaction is balanced. Conjugate redox pair refers to the acceptor and donor of a half reaction. Formation of Hydrogen fluoride.5: Redox Reactions.Most oxidation-reduction (redox) processes involve the transfer of oxygen atoms, hydrogen atoms, or electrons, with all three processes sharing two important .
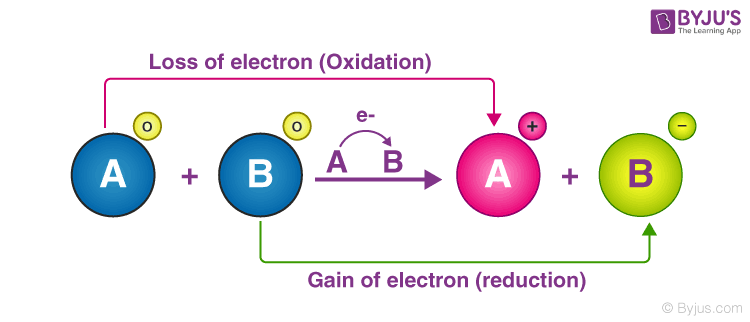
The reactants are elements, and it is assumed that they are electrically neutral; they have the same number of electrons as protons.Oxidation-reduction (redox) reactions are those in which one or more elements involved undergo a change in oxidation number.An additional example of a redox reaction, the reaction of sodium metal with chlorine is illustrated in Figure \(\PageIndex{1}\). Reaction of Iron and copper sulphate.Redox reactions are often balanced by balancing each individual half reaction and then combining the two balanced half reactions.Oxidation half-reaction: Al → Al3+ + 3e− Al → Al 3 + + 3 e −.The net balanced redox reaction is as follows: Al + 3Ag + → Al 3 + + 3Ag.If so, it is an oxidation/reduction reaction or Redox Reaction for short.Redox Reaction Examples. The balanced equation is the sum of the two half-equations adjusted to equalize the . Note the transfer of electrons from Fe to Cl.An example of redox titration is the treatment of an iodine solution with a reducing agent.And this is to remember that losing an electron means you are being oxidized, or losing electrons is oxidation.Oxidation and Reduction reactions- The chemical reactions which involve the transfer of electrons from one chemical substance to another. One example of this is the smelting of metal sulfide with a reducing agent present. Reaction of Zinc and copper sulphate.Since the hydrogen ions gain electrons, it is a reduction reaction. so, write a balanced equation for the reaction. Assigning Oxidation States.(Note: the oxidizing and reducing agents can be the same element or compound, as in disproportionation reactions discussed below).Redox reactions are utilized in the process of extracting metals from their ores.Examples of Reduction.Biology definition: Redox reaction is a chemical reaction involving both reduction and oxidation, which results in changes in the oxidation numbers of atoms included in the reaction. The product, however, is ionic; it is composed of Na + and Cl − ions. The hydrogen is .What is a redox reaction with an example? Solution.) 20: Electrochemistry. To identify oxidation–reduction reactions in solution. In step#1, the catalyst absorbs H − H and RR ′ C = CR R ′ on the surface, as illustrated in Figure 4.Consider an example of the combustion redox reaction between magnesium (Mg) and oxygen (O 2 ), resulting in magnesium oxide .1 “Standard Reduction Potentials of Half Reactions” and change the sign on the E ½ value. Reduction is the gain of electrons.
Oxidation-Reduction Reactions
5: Oxidation-Reduction (Redox) Reactions is shared under a CC BY-SA license and was authored, remixed, and/or curated by LibreTexts. The term ‘redox’ is formed by combining reduction and oxidation. To make the oxidation reaction, simply reverse the reduction reaction in Table 14. You will also learn the difference between oxidation and reduction, and the definition of oxidation. A good example of a redox reaction is the .General Chemistry. The following example demonstrates this process.The reduction agents in the previous examples are: H2, HgCl2, and C.The chemical reaction that happens within a battery which powers your phone or laptop devices, this is also an example of redox reaction. The burning of organic material and . Many oxidation-reduction reactions are as common and familiar as fire, the rusting and dissolution of metals, the browning of fruit, and respiration and . It involves that there should be loss of electrons by one element and gain by another elements, both which are part of the chemical reaction.
Another example of a redox reaction is this combination (synthesis) reaction between magnesium and chlorine. A redox reaction is utilized in the production of gold-plated ornaments to deposit a thin layer of material onto the object’s surface.

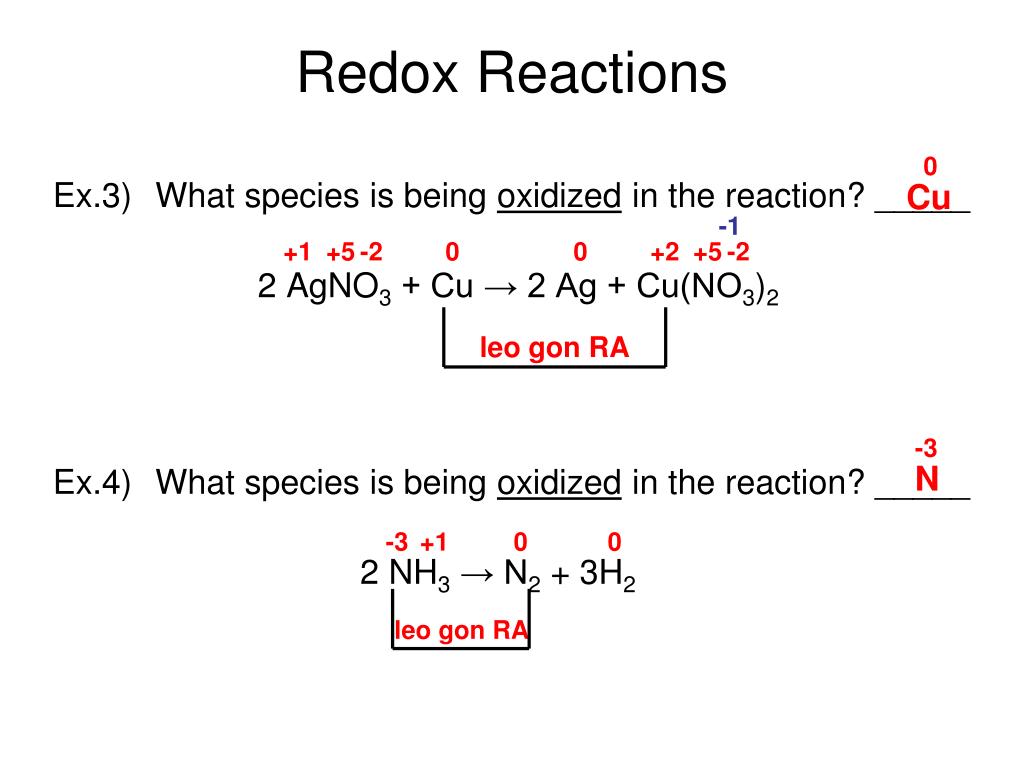
To combine these two half reactions and cancel out all the electrons, we need to multiply the silver reduction reaction by 3: Now the equation is balanced, not only in terms of elements but also in terms of charge.
Oxidation-reduction reaction
Geschätzte Lesezeit: 8 min So let’s start by looking at the reaction that forms table salt. There is still only one Al atom on each side of the chemical equation, but there are now three Ag atoms, and the total charge on each side of the equation is the same (3+ for both sides).Every redox reaction consists of two half reaction, where one substance donates electrons and thus becomes an oxidized product while another substance accepts the electrons and thus becomes a reduced product. Reaction of Copper sulphate and potassium iodide. Oxidation is the loss of electrons. In fact, the type of chemical reaction that happened within you to break down sugar and give you energy to run and play is also an example of redox reaction.1: Oxidation States and Redox Reactions.Geschätzte Lesezeit: 5 min
Redox

If the reduction potential is negative, make the voltage for the oxidation positive; if .
Reduction Definition and Examples in Chemistry
These are called half-reactions because they make up half of a full redox reaction: Cu 2+ (aq) → Cu (s) F 2 (g) → 2F – (g) What is Oxidation? Oxidation Definition: .
Redox Titration
Oxidation-reduction reactions are vital for . Oxidation and reduction are two simultaneous processes that occur together, and they result in a very famous reaction, known as: redox reaction. So far we have covered two types of reactions that must involve oxidation and reduction, the single displacement reaction and the combustion reaction. The surface-bonded species can migrate along the surface. The burning of fuels that provides the energy to maintain our civilization . The endpoint of this titration is detected with the help of a starch indicator.Recognize a reaction as an oxidation-reduction reaction. Learn for free about math, art, computer .Combustion forms the classic example of redox reactions in real life. Reduction Potential of a Half-Reaction. These electron-transfer reactions are termed as oxidation-reduction reactions or Redox reactions. Notice that the chloride ion #(Cl^-)#, was not involved in either the oxidation or reduction. Electron transfer is one of the .
A few examples of such reactions will be used to develop a clear picture of this classification. Predict whether iron(III) ion, Fe 3 +, will oxidize copper metal. A substance that loses electrons is said to be oxidized, and the substance that gains electrons is said to be reduced.Overview
Redox Reactions
The term oxidation was originally used to describe chemical reactions involving O 2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions.Let’s look at some examples of reduction half-reactions. The easiest way to fully understand redox reactions is to look at some examples.In this article, we are going to see, what redox reaction example is with their explanation in detail. Equations for redox reactions can be produced by adding together the two ion-electron equations . Redox reactions can be used in electrochemical cells to produce electricity. The term covers a large and diverse body of processes.During the process of respiration, the carbon dioxide is reduced whereas the water is oxidized to form oxygen.Thus, redox reaction can be defined as the reaction in which both oxidation and reduction takes place simultaneously.Example of Redox Reactions.Geschätzte Lesezeit: 6 min
Oxidation-Reduction Reactions
A few examples of redox reactions, along with their oxidation and reduction half-reactions, are provided in this subsection. Oxidizing and Reducing Agents.Oxidation-reduction reactions are of central importance in organic chemistry and biochemistry. Oxidation and Reduction Reaction.The electron transfer system in cells and oxidation of glucose in the human body are examples of redox reactions.The formation of hydrogen fluoride is an example of a redox reaction. A chemist can atom balance and charge balance one piece of an equation at a time. The term oxidation was first used to describe reactions in which .Chemical reactions in which electrons are transferred are called oxidation-reduction, or redox, reactions. H ′ s and the π -bond of alkene become bonded with the catalyst surface. Oxidation is when . While the vast majority of redox reactions involve changes in oxidation number for two or more elements, a few interesting exceptions to this rule do exist as shown below\). We can break the reaction down to analyze the oxidation and reduction of reactants. Sodium is oxidized and .1 lists only reduction reactions, but a redox reaction has a reduction and an oxidation. This is because it neither gained nor lost electrons during the reaction. RA: CH3OH OA: O2. Consider this chemical reaction: 2Na (s) + Cl 2 (g) → 2NaCl.Redox reactions. The substance oxidized is the reactant that had undergone . Reduction is the addition of Hydrogen or removal . So lets understand more about these reactions.

Oxidation-Reduction or redox reactions occur when elements in a chemical reaction gain or lose electrons, causing an increase or decrease in . In step#2, H − H -bond breaks.Mechanism of catalytic hydrogen of alkenes. 2 SO 2 + O 2 → 2 SO 3 ; CuSO 4 + 2 AgNO 3 → Ag 2 .Example \(\PageIndex{1}\): Oxidation.At the end of an article is a video showing a great experiment for budding middle school chemists.

Redox Reactions: Definition, Types, Balancing, Applications
Example 1: Reaction between Hydrogen and Fluorine In the reaction between hydrogen . Oxidation and reduction always occur together, even though they can be written as separate chemical equations.Which reactions are redox reactions? For those that are redox reactions, identify the oxidizing and reducing agents. Example: Cu 2 + (aq) + Zn (s) → Cu (s) + Zn 2 + (aq) 2Cu2O (s) + Cu2S (s) → 6Cu (s) + SO2(g) Oxidizing or reducing agent in a chemical reaction, is typically identified by its oxidation . Reaction of Hydrogen sulphide and . The H + ions, with an oxidation number of +1, are reduced to H 2, with an oxidation number of 0, in the reaction : Zn (s) + 2H + (aq) → Zn 2+ (aq) + H 2 (g) Another simple example is the reaction between copper oxide and magnesium to yield copper and magnesium oxide: Rusting of iron is a process that . Expand/collapse global location. In all oxidation–reduction (redox) reactions, the number of electrons lost equals the number of electrons gained.A reaction in which there is a transfer of electrons is said to be an oxidation-reduction reaction, or a redox reaction. Another one that’s often used is OIL RIG. And this, essentially– oxidation is losing electrons, reduction is gaining electrons. Assigning oxidation states to the elements in binary ionic compounds is straightforward: . The definitions for oxidation and reduction are: Oxidation: the loss of electrons Reduction: the gain of electrons.Examples of Redox Reactions.
Redox (Oxidation-Reduction) Reaction: Definition
And gaining electrons is reduction. Decomposition is also a way to simplify the balancing of a chemical equation.RA: H2 OA: H2O2.The most common oxidation-reduction (redox) reactions are combination, decomposition, displacement, and combustion reactions.oxidation-reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes.This is an example of a redox reaction; a chemical reaction in which the oxidation numbers of elements change on going from reactants to products. A substance can be either an electron donor or an electron . C (s) + O 2 (g) → CO 2 (g) . However, whenever we talk about combustion, we usually view it as a physical change than a chemical one.For example, in the above reaction, it can be shown that this is a redox reaction in which Fe is oxidised, and Cl is reduced. Map: Chemistry – The Central Science (Brown et al.
What is a reduction reaction? + Example
Definitions for the complementary processes of this . Combustion forms the classic example of redox reactions in real life. The oxidation and reduction reaction also involve the addition of oxygen or hydrogen to different .
- What Is Clay Material | Use of different types of clay in construction
- What Is Diabetic Nephropathy _ Diabetic Kidney Disease
- What Is A Transgender Person Called?
- What Is A Morph Cut Transition?
- What Is An Api Oil–Water Separator?
- What Is A Sacrificial Anode? – Sacrificial Anodes FAQs
- What Is An Example Of A Viola?
- What Is An Atm Machine _ NCR Atleos
- What Is Cordierite | Pizzastein Material Check vom Profi
- What Is An Ncvr (Non-Crate Very Rare)?
