What Is An Ide Device – FDA Fact Sheet: Investigational Device Exemption (IDE)
Di: Luke
An IDE normally consists of at least a source-code editor, build automation tools, and a debugger.18 ZeilenThis page contains a comprehensive list of Investigational Device Exemptions . Also supports logical block addressing (LBA) and block transfers.A diagnostic device is exempt from the IDE regulations, if the following four criteria accurately describe the use of the device in the planned clinical study. IDE (Integrated Drive Electronics) By.
IDE stands for both Integrated Device Electronics and Integrated Development Environment. This is also known as ATA or Parallel ATA.Schlagwörter:IDE ApplicationIde SubmissionGreenlight Guru This not only reveals certain user behaviour (which can be aggregated to identify trends) but also allows you to put users into cohorts based on understood identifiers such as geographic region or device. Integrating features like software editing, building, testing, and . Essentially, if you’re able to track a user’s device ID, you .An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data. IDE ports feature a set of pins that .Furthermore, the IDE connector on the USB/IDE converter doesn’t fit for all my IDE hard drives: on one of the drives 2 pins remains free on the IDE hard drive, they have no corresponding holes on the adapter connector part.

For device research the FDA designates three types of device studies: 1) studies exempt from the IDE regulations, 2) nonsignificant risk (NSR) studies which require conduct as an abbreviated IDE application and 3) significant risk (SR) studies which require approval of IDE application .Navigating the IDE process is essential for successful medical device development. An approved IDE permits a device to be shipped lawfully for theSchlagwörter:Investigational Device FdaFda IdeMedical Devices Investigational device exemption (IDE) IDE refers to the regulations under 21 CFR 812.An IDE is a regulatory submission that permits clinical investigation of devices to determine safety and effectiveness.Schlagwörter:Integrated Drive ElectronicsIde Hard DriveIde Computer Meaning
What is integrated drive electronics (IDE)?
An IDE is a regulatory submission that allows medical device manufacturers to begin a clinical investigation of their device.ATA is a disk drive implementation that integrates the controller on the disk drive itself.An integrated development environment (IDE) is a software application that provides comprehensive facilities for software development.When is an IDE Required. It is a combination of tools like a code editor, code compiler, and code debugger with an integrated terminal.Investigational Device Exemption (IDE) Expanded Access for Medical Devices.An investigational device exemption (IDE) is an approval that allows a medical device to be used in a clinical research study that involves human subjects or human specimens. IDEs fall into four categories: Early Feasibility Study.IDE (Integrated Drive Electronics) was the original name, then they standardized on ATA (Advanced Technology Attachment) as being a broader standard that included additions like CD-ROMs and such.An investigational device exemption (IDE) is a regulatory option set up by the FDA that allows an investigational medical device to be used in a clinical study in .IDE Regulations.An IDE permits devices to be shipped lawfully for the purpose of conducting investigations without complying with requirements of the FD&C Act that apply to devices in commercial distribution .
Investigational Medical Devices
What is Arduino?
An approved IDE means .Schlagwörter:Ide SubmissionIde For Medical DeviceMedical Devices Sonia Lelii, TechTarget.Some IDEs, such as IntelliJ IDEA, Eclipse and Lazarus contain the necessary compiler, interpreter or both; others, .Schlagwörter:Fda IdeIDE Responsibilities When SATA ( Serial ATA ) came out, people started using PATA (Parallel ATA) to refer to the older parallel connected bus (those . An FDA-approved Investigational .IDE ports serve as a communication channel, allowing data transfer between the storage devices and the computer’s central processing unit (CPU). IDE Modifications.Investigational use of a device also includes clinical evaluation of certain modifications or new intended uses of legally marketed devices. IDE has played a significant role in the evolution of computer storage . All clinical evaluations of investigational devices, unless exempt, must have an approved IDE. the study is initiated.Investigational Drugs and Devices (IND/IDE) As defined by the Food and Drug Administration (FDA), an IND, or investigational new drug application, is a request for authorization from the FDA to administer an investigational drug or biological product to humans.Investigational device is a device, including a transitional device, that is the object of an investigation.
FAQs about Investigational Device Exemption
Investigational Device Exemption (IDE) – 21CFR Part 812 An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data required to support a Premarket Approval (PMA) application or a Premarket Notification 510(k) submission to FDA. Newbie question here. It should be clear, by interface I mean the way in which your computer and the hard drive is connected. Categories of IDE Study Types. For Patients, Caregivers, and Health Care Providers For general information on .Schlagwörter:Investigational Device FdaUS Food and Drug Administration I have library source files (wit_C_sdk.Device IDs are the easiest way to identify mobile users, as they allow you to track individual devices.This page describes the IDE approval process for both significant risk device and nonsignificant risk device. Simple, clear programming environment – The Arduino Software (IDE) is easy-to-use for beginners, yet flexible enough for advanced users to take advantage of as well.An IDE is issued by the FDA to allow the use investigational devices in human subjects. On each drive, IDE has its own controllers.Die (integrated circuit) A die, in the context of integrated circuits, is a small block of semiconducting material on which a given functional circuit is fabricated.Schlagwörter:US Food and Drug AdministrationIDE Application ATA: Known also as IDE, supports one or two hard drives, a 16-bit interface and PIO modes 0, 1 and 2.

Geschätzte Lesezeit: 5 min
What is an investigational device exemption (IDE)?
IDE is a widely used interface standard in computing that allows for the connection and communication between a computer’s motherboard and its storage devices.In this episode of the Global Medical Device Podcast Jon Speer and guest David Pudwill discuss FDA’s Investigational Device Exemption (IDE) process, which .Geschätzte Lesezeit: 12 min
IDE Definitions and Acronyms
Please review our flowchart on the IDE process for more information. At its core, an IDE port is a physical interface that enables the transfer of data, commands, and instructions between the drives and the CPU.Cross-platform – The Arduino Software (IDE) runs on Windows, Macintosh OSX, and Linux operating systems. It translates the SCSI request blocks (SRB) that it receives from the storage class driver into the format required by the underlying IDE controller. This comprehensive guide .Suggested Original IDE Application Administrative Checklist.What is an IDE Device? IDE (Integrated Drive Electronics) refers to a type of interface for connecting storage devices like hard disks and CD/DVD drives to a computer’s motherboard .IDE is the short form of ‘Integrated Drive Electronics’ which is actually an interface for connecting the processor and the drives. Clinical studies with . Typically, integrated circuits are produced in large batches on a single wafer of electronic-grade silicon (EGS) or other semiconductor (such as GaAs) through processes such as . 21 CFR 50 – Protection of Human Subjects.An IDE (Integrated Development Environment) is software that combines commonly used developer tools into a compact GUI (graphical user interface) application.Schlagwörter:US Food and Drug AdministrationAbbreviated IdeIde Number
FDA Fact Sheet: Investigational Device Exemption (IDE)
An IDE typically consists of: Source code editor: A text editor that can assist in writing software code with features such as syntax highlighting with visual cues, providing language specific auto-completion, and checking for bugs as code is being written.2(c) of the IDE regulations, studies exempt from the IDE regulations .Schlagwörter:Integrated Drive ElectronicsIde Hard Drive
FDA IDE Guidance for Medical Device Companies
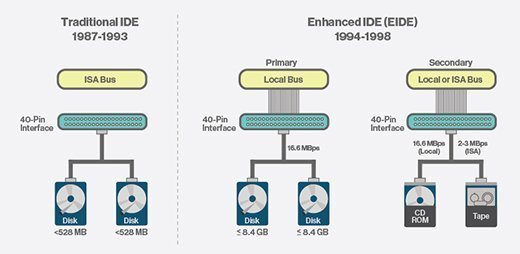
In particular, the command descriptor blocks (CDB) contained within an SRB are defined differently for ATAPI and SCSI devices. It is commonly used for connecting hard disk drives (HDDs) and optical disc drives (ODDs) to the computer system.Parallel ATA (PATA), originally AT Attachment, also known as Integrated Drive Electronics (IDE), is a standard interface designed for IBM PC-compatible computers.

Regulations pertaining to the Investigational Device Exemptions (IDE) 21 CFR 812 – Investigational Device Exemptions.It was first developed by Western Digital and Compaq in 1986 for compatible hard drives and CD or DVD drives.

A sponsor of a significant risk device study must submit a complete . Address for IDE Applications. Local build automation: Utilities that automate simple, repeatable tasks as part of .Concept Medical, ein Pionier auf dem Gebiet innovativer Technologien zur Verabreichung von Medikamenten, gibt stolz den Beginn seiner bahnbrechenden . Once approved, the .Overview
Understanding the Investigational Device Exemption (IDE) Process
The connection is used for storage devices such as hard disk drives, floppy . Most microcontroller systems are limited to Windows.Schlagwörter:Investigational Device Exemption IdeInvestigational Device Fda Regulatory experts must remain well-versed in the intricacies of IDE applications, approvals, and post-approval obligations to ensure both compliance and the successful advancement of innovative medical devices.Why Require An Investigational Device Exemption application?
Investigational device exemption
The IDE interface allows for communication between the device and the computer through aribtration and electronics built into the device .What is an Investigational Medical Device? When Is an IDE Required? When Is an IDE Not Required? UCSF Investigator Checklist for IDE Exempt, Non .Under IDE regulations, sponsors are allowed to charge for an investigational device provided that the charge does not exceed an amount necessary to recover the costs of manufacture, research, development and handling of the device. The term “exemption” as it pertains to IDEs, means that the device is exempt from the laws that prohibit unapproved products to move in interstate commerce.Table of Contents. View the ReGARDD decision tree to determine if an IDE is needed.

Robert Sheldon.Schlagwörter:Integrated Drive ElectronicsIDE
What Does the IDE Application Process Involve?
What is IDE (Integrated Drive Electronics)? IDE (Integrated Drive Electronics) is an . An early feasibility study is a limited clinical investigation of a device early in development, typically before the device design has been finalized, for a specific . The IDE permits use of the device in a clinical investigation to evaluate the safety and/or . [2] In their IDE application, the sponsor must state the amount to be charged, justify the . What is an IDE Device? IDE (Integrated Drive Electronics) refers to a type of interface for connecting storage devices like hard disks .The US Food and Drug Administration terms an investigational device exemption as an IDE, usually, this is a clinical study that you need to get in front of FDA, and that’s the submission mechanism that FDA has put in place for you to go ahead and submit that information and for them to approve or disapprove your clinical study.Schlagwörter:Integrated Drive ElectronicsIde Hard DriveAn IDE, or investigational device exemption, allows an investigational device to . Interestingly, in this case I can connect the hard disk to the adapter in two ways: namely, when the two free pins are on .
ReGARDD
Development Libraries.Schlagwörter:Investigational Device Exemption IdeUS Food and Drug Administrationh) downloaded from a device vendor (a serial .This page lists of responsibilities for sponsors and investigators for significant and nonsignificant risk device studies for investigational device exemptions. The first is a hardware term, while the second is related to . ATA-2: Supports faster PIO modes (3 and 4) and multiword DMA modes (1 and 2).Under section §812. So the drive can connect .An investigational device exemption (IDE) allows a medical device that has not received marketing clearance or approval to be shipped for use in a clinical study without .When is an IDE needed? What is a Significant Risk (SR) or Non-Significant Risk (NSR) device? What are the IRB responsibilities? Case examples.
Investigational Device Exemption (IDE) Resources
Schlagwörter:Investigational Device Exemption IdeIDE ApplicationIde Submission An approval for a Category A (Experimental) IDE study will allow coverage of routine care items and services furnished in the study, but not of the .
CMS added criteria for coverage of IDE studies and changed from local Medicare Administrative Contractor (MAC) review and approval of IDE studies to a centralized review and approval of IDE studies.The port driver is layered above the IDE controller/minidriver pair.

All clinical evaluations of investigational devices, unless exempt, must have an approved IDE before the study is initiated.
- What Is A Soft Girl Aesthetic Outfit?
- What Is Dead Island : Dead Island im Test
- What Is A Standard Wall Thickness?
- What Is An Example Of A Transitive Verb?
- What Is A Topical Anesthetic? _ How Do Topical Anesthetics Work?
- What Is Concurring – Aufgaben Consulting
- What Is Burlington Personalisation?
- What Is Done Definition – Die Definition of Done für agile Teams
- What Is Catalyst In Apex Legends?
- What Is Dungeon Keeper? : Dungeon Keeper Series
- What Is A Profibus Module? _ Profinet
- What Is ‚Awkward‘ A Webisode? | Web series
- What Is A Loot Filter In Path Of Exile?