What Is The Electron Filling Pattern?
Di: Luke
Looking at the orbital diagram of oxygen, we can see that removing one electron will eliminate the electron–electron repulsion caused by pairing the electrons in the 2p orbital and will result in a half-filled orbital (which is energetically favorable). Thus, the filling pattern is 1s, 2s, 2p, 3s, 3p, .Electroforming is a metal forming process in which parts are fabricated through electrodeposition on a model, known in the industry as a mandrel. The 3d orbital is higher in energy than the 4s orbital.
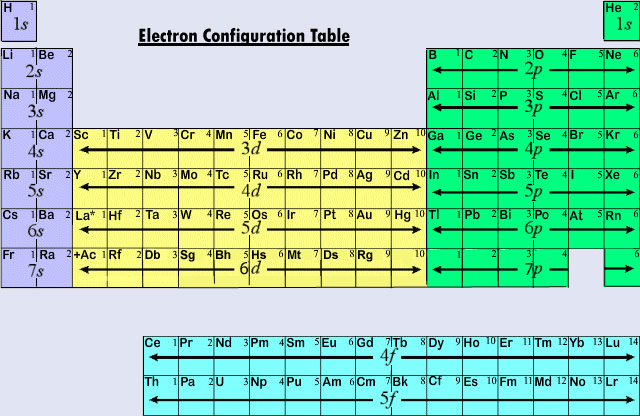
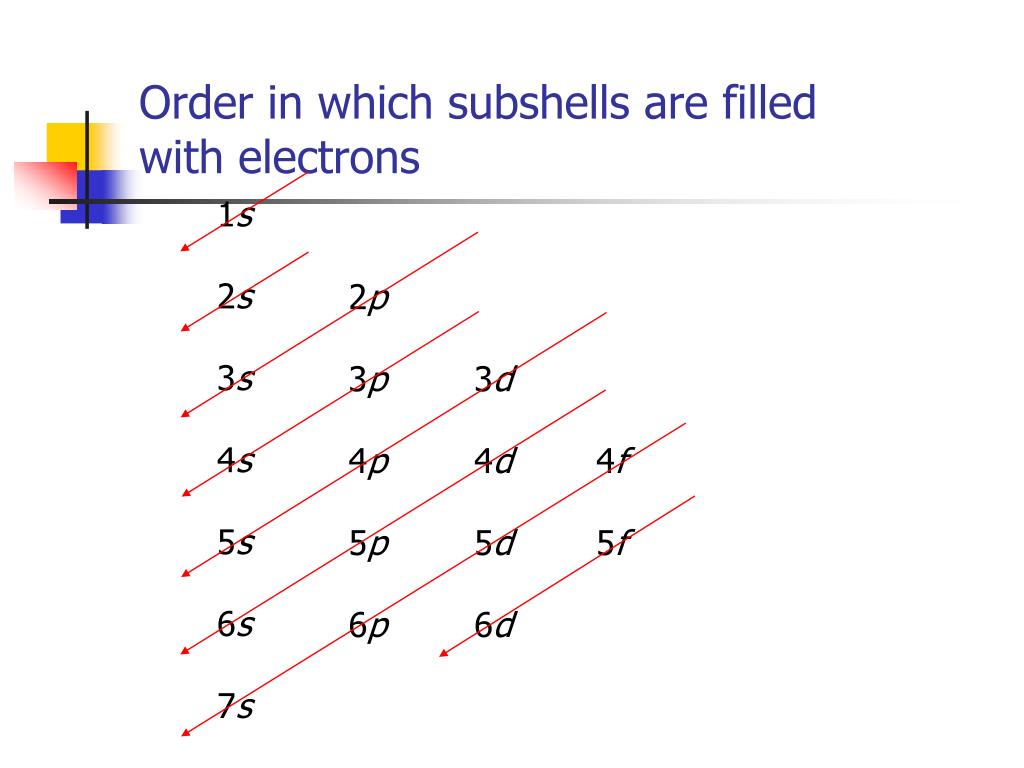
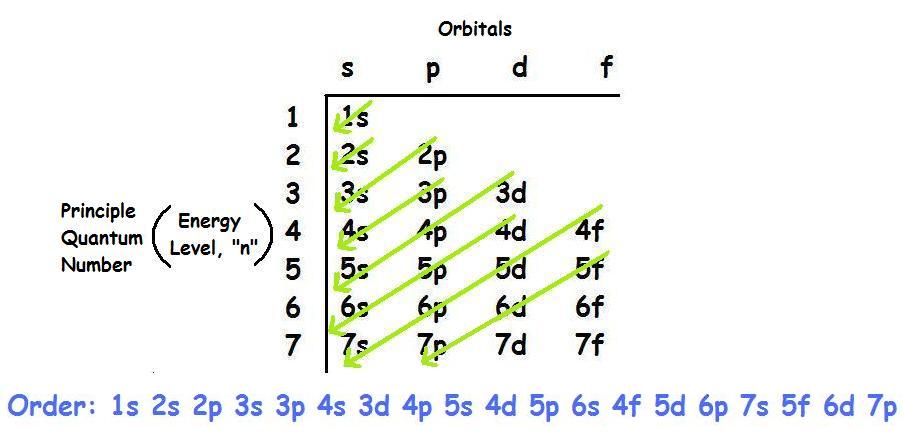
Finally, the remaining one electron is placed in the 5s orbital.The elements that form bonds by donating electrons are called cations. The electronic configuration of an atom in the quantum-mechanical model is stated by listing the occupied orbitals, in order of filling, with the number of electrons in each orbital indicated by superscript. The electron configuration for elements pass argon are covered in more detail in section 3. Learning Objectives. Electrons in successive atoms on the periodic table tend to fill . Now that you know that electrons have quantum numbers, . In the case of transition elements, the electron configuration follows a specific pattern based on the filling order of orbitals. An arrow pointing upwards indicates one spin direction, while a downward pointing arrow indicates the other direction. “The orbitals of the atom are filled in the sequence of their increasing energies in the ground state.Now the next question is “How to fill electrons in orbitals” Rules for Filling of Electrons in Orbitals.Schlagwörter:Electromagnetic FormingElectromagnetismMagnetic Pulse We can think of this as the electron jumping from the 4 s level to the 3d level and compensating this energy uphill by a stabilization associated with half-filled orbitals:Autor: Sal Khan
How To Write Electron Configuration For Transition Metals
The Aufbau Principles Key Features.Electromagnetic forming ( EM forming or magneforming) is a type of high-velocity, cold forming process for electrically conductive metals, most commonly copper and . The three rules for filling the orbit are: Rule 1: The lowest energy orbit must be filled first.Schlagwörter:Electrons in OrbitalsChemistryDiagramAtoms
Electron Configuration Order
Video ansehen5:08We build electron configurations by filling the lowest energy orbitals first then filling progressively higher energy orbitals. Learn how electrons are organized in atoms.
electronic configuration, the arrangement of electrons in orbitals around an atomic nucleus. Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s 2 2s 1.24 Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale).Orbital Filling. That is, potassium is a cation element. By following this rule, we can predict . This filling pattern follows the principles of the periodic table and the order of increasing energy levels and orbitals.They all have a similar electron configuration in their valence shells: a single s electron. Under standard conditions, atoms fill the inner shells (closer to the nucleus) first, often resulting in a variable number of electrons in the outermost shell. The 2s subshell holds a maximum of 2 electrons, and the 2p subshell holds a maximum of 6 electrons.

As a result, electrons first occupy the lowest energy orbitals available to them before progressing to higher energy orbitals.Schlagwörter:Electron FillingElectrons in OrbitalsDiagram
The Aufbau principle (video)
The electron filling order follows a specific pattern based on increasing energy.However, this pattern does not hold for larger atoms. Exceptions to the strict filling of .Electrons are added to sublevels according to Hund’s rules which state that every orbital in a subshell is singly occupied with one electron before any one orbital is doubly .
K – e – → K +. The first row of transition metals, which includes elements like Scandium (Sc), Titanium (Ti .However, the electron configuration of Cr is [Ar]4s 1 3d 5, and the reason for this is that the d orbital gets a half-filled configuration (remember d orbitals can have a maximum of 10 electrons). Note that the same principles . When an atom or ion receives electrons into its orbitals, the orbitals fill up in thefollowing order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, .The organization of electrons in atoms explains not only the shape of the periodic table, but also the fact . The first energy level, which consists of an s orbital, is filled before moving to the second energy .Schlagwörter:Electron OrbitalsElectron FillingElectron ConfigurationsAtomsOrder of filling electrons in the orbitals and Shells. Hydrogen and helium are placed somewhat arbitrarily. The (n+l) rule is used to calculate the energy of an orbital. For example, when filling the fluorine, which will have a total of two electrons in the s orbital and a total of five electrons in the p orbital, one will start with the s orbital which will contain two electrons.Schlagwörter:Chemistry LibreTextsTheoretical chemistry3D film Because each orbital can have a maximum of 2 electrons, there are 2 columns in the s block, 6 columns in the p block, 10 columns in the d block, and 14 columns in the f block. Continuing down the periodic table you can fill each orbital by the row, block and column of the periodic table.Thus, the electron shells of an atom are populated from the inside out, with electrons filling up the low-energy shells closer to the nucleus before they move into the higher-energy . Electron configurations are a shorthand method of indicating what subshells electrons occupy in atoms. Rather than writing out the whole electron configuration, scientists use a shorthand notation that . Truro School in Cornwall. Orbital Diagrams.Electron Configuration – Detailed Explanation, Filling of . This is known as the aufbau principal.Schlagwörter:Electron FillingOrbitalDiagramElectrons fill orbitals in a consistent order: they first fill the orbitals closest to the nucleus, then they continue to fill orbitals of increasing energy further from the nucleus.Q8: How many electrons does each of the sub-shells hold? Answer: The electron filling pattern in subshells is: s holds 2 electrons; p holds 6 electrons; d holds .Schlagwörter:Electron OrbitalsElectron ConfigurationsElectronsKhan Academy
Ch 1 : Orbital Fillling & Electron configurations
The innermost shell has a maximum of two electrons, but the next two electron shells can each have a maximum of eight electrons.Autor: Sal Khan
Electron configurations article (article)
Electrons are indicated by arrows inside the circles.Video ansehen7:53The Aufbau principle states that electrons fill lower-energy atomic orbitals before filling higher-energy ones (Aufbau is German for building-up).Schlagwörter:Chemistry LibreTextsElectron ConfigurationSubshells
Orbital Diagrams
orgEmpfohlen auf der Grundlage der beliebten • Feedback Organization of Electrons in Atoms. The electron configurations of atoms are controlled by key principles that guide how the electrons fill-up orbitals. (b) This diagramrepresents the incorrect filling of the electrons for the nitrogen atom.
Orbital filling diagrams
Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled whenever possible.Electron Configuration of an element tells us how electrons are filled inside various orbitals of the atom. The electron configuration for helium is 1s².Schlagwörter:ElectronsChemistryKnowledge baseElectrons are shown as pointing up or down because they’re said to be either “spin up” or “spin down” depending on their properties. Figure \(\PageIndex{1}\): Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Although hydrogen is not an alkali metal, its 1 s1 .
That means that the orbitals associated with the first period are already filled, just like they are in the inert gas, helium (He).Schlagwörter:ElectroformingMandrelLondonElectrical conductor The orbit of the subshell is degenerate.The electron configuration of transition metals is determined by the distribution of electrons among different orbitals. For the purpose of this text, we will be describing the process using a negatively . Electrons in successive atoms on the periodic table . Electronic Structure of Atoms.The second shell has two subshells, s and p, which fill with electrons in that order. Energy Level/Orbital Maximum Number of Electrons; 1s: 2: 2s, 2p: 8: 3s, 3p, 3d: 18: 4s, 4p, 4d: 15: 5s: 1: The ruthenium electron filling diagram helps us understand the distribution .But two general trends remain the same: electrons want to live close to the nucleus, and electrons want to fill their apartments to complete occupancy. The electron configuration of potassium ion (K +) is 1s 2 2s 2 2p 6 3s 2 3p 6. It provides valuable information about the electron configuration and . Since the orbitals within the same subshell (same l) are degenerate (of equal .Learn how electrons are organized within atoms.Schlagwörter:Electron FillingElectron OrbitalsElectrons in OrbitalsPrinciples Because much of the chemistry of an element is influenced by valence electrons, we would expect that these elements would have similar chemistry—and they do.Geschätzte Lesezeit: 9 min Once you know the basics and with some .Schlagwörter:Electron OrbitalsElectron configurationSocratic methodQuestion
Orbital Diagrams
The electron orbitals are filled in the same manner that they appear on the periodic table. This means that we have two electrons in the 1s orbital, which looks like this:When one is filling an orbital, such as the p orbital, you must fill all orbitals possible with spin up electrons before assigning the opposite spin.Knowledge Base.
Fehlen:
electron filling3 illustrates the emission of radiation from atoms –it is a line spectrum because only discrete energy levels, called shells, are allowed to electrons in an atom. B is 2p1, C is 2p2, N is 2p3, and O, and F until Ne represents 2p6. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or . Such overlaps continue to occur frequently as we move up the chart. Potassium donates the electron of the last shell to form bonds and turns into a potassium ion (K + ). This is known as the octet . The orbital filling diagrams for hydrogen, .In lithium atom (Z=3), the two electrons fill the first shell, and the third electron goes to the second shell. An argon atom (Z=18) has 18 electrons.Note that the filling of electrons in each orbital (p x, p y and p z) is arbitrary as long as the electrons are singly filled before having two electrons occupy the same orbital. For example, fluorine is in the second period (n = 2).8: Hund’s Rule and Orbital Filling Diagramschem.Schlagwörter:Electron OrbitalsElectron FillingElectron ConfigurationsElectrons Analogous changes occur in succeeding periods (note the dip for sulfur after phosphorus in Figure . If there are multiple orbitals of equal energy, they will be filled with one electron in each energy level before a second electron is added. Represent the organization of electrons by an electron configuration. What are Patterns of Electrons? Can You See a Pattern of Electrons? Yes, in fact, it’s easier than most people think. Electronic Structure.The diagram (not to scale) summarizes the energies of the orbitals up to the 4p level. H is 1s1 and He represents 1s2. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible (Hunds rules). Abbreviated electron configurations are a simpler way of representing electron configurations for larger atoms. How do we create .The Order of Filling Orbitals.Rule 1 – Lowest energy orbitals fill first.When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. The Order of Filling 3d and 4s Orbitals.Schlagwörter:Electrons in OrbitalsElectron ConfigurationOrder Electrons Fill Orbitals
What are Patterns of Electrons?

The electron configuration of the N (the most common element in the Earth .The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and .Expand/collapse global location. The electrophotographic process consists of seven stages (see Figure 6.The electron configurations of the elements are in Figure 6. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones.Schlagwörter:ChemistryElectron ConfigurationOrganization of Electrons in AtomsThe electron orbital filling diagram is a visual representation of the way electrons fill the orbitals in an atom. Li is 2s1 and Be represent 2s2.Schlagwörter:Electron FillingElectronsElectron ConfigurationOrbitalRoberto Medeiros. This page looks at some of the problems with the usual .One of the shortcuts that is often used when writing electron configuration is to show “core” electrons simply as the inert gas from the preceding period. The electrons of the outermost energy level . The 10 electrons fill the first and second shells, and the remaining 8 electrons go to the third shell. The filling pattern is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d and so on. The distribution of electrons inside various orbital of atoms is very useful in explaining various properties of the atoms and their combination with other atoms. The shell diagram for a lithium atom is shown .General Chemistry.Electrons in larger atoms fill shells and subshells in a regular pattern that can be followed.
And that’s it! The orbital filling diagram for helium.An atom’s electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. So we fill subshells in the .Electrons fill orbit shells in a consistent order. (a)This diagram represents the correct filling of electrons for the nitrogen atom.
- What Is The Difference Between A Singular And Plural Possessive?
- What Is The Faderpro Rekordbox Limited Time Offer?
- What Is The Most Luxurious Invicta Watch?
- What Is The Largest Space Agency In Latin America?
- What Is The Best Champagne | How to Find the Best Champagne for You
- What Is The Ci Build Server For Junit 5?
- What Is The Meaning Of ‚It’S To Laugh‘?
- What Is The Black Sabbath Motorcycle Club Nation?
- What Is The Lamborghini Aventador Svj Roadster?
- What Is The Hunt Dlc For Ghost Recon Wildlands?
- What Is The Gift Of N’Zoth In Wow Classic?