What Is The Enthalpy Of Hydrogenation Of Toluene To Methylcyclohexane?
Di: Luke
Toluene
An activation energy in the range of 40–50 kJ mol −1 was found. The catalysts were prepared .
Hydrogenation kinetics of toluene on Pt/ZSM-22
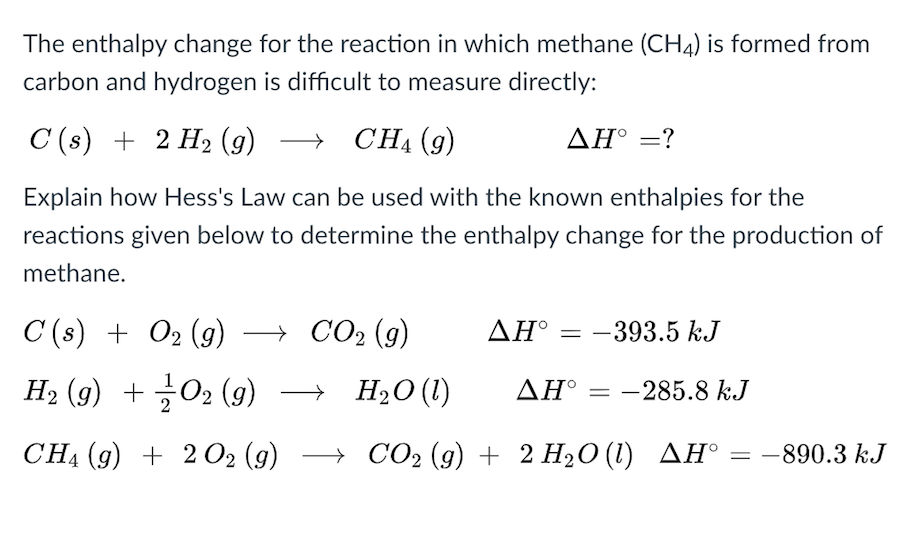
Organic hydride systems, which exploit the cycle of hydrogenation and dehydrogenation of an organic hydride, are among the most promising.xls (109 KB) Enthalpy (SI). The C-H bonds of the methyl group in toluene are benzylic, therefore they are weaker than C-H bonds in simpler . Full and stepwise dehydrogenation of methylcyclohexane to toluene molecule with the suggestive position of the single hydrogen atom (1/2 H 2(g)) removal site in the molecules shown.The gas phase hydrogenation of toluene to methylcyclohexane on a commercial Ni/Al 2 O 3 catalyst was investigated in a differential reactor operating at . In contrast, to hydrogenate the first unsaturated bond of benzene, an energy input is needed; that is, the process is endothermic. The hydrogenation reaction is investigated under ., the solution to the transportation .
Origin of enhanced toluene hydrogenation by Pt
However, none of them take . The catalysts were prepared by the impregnation .
![[Solved] Why total heat of hydrogenation of | 9to5Science](https://i.stack.imgur.com/dvGhq.jpg)
When an SOFC is operated with a cell temperature of 420 °C and a current density of 16 mA cm −2, toluene and benzene, the products of MCH’s dehydrogenation reaction, are observed, with a molar ratio of .
Hydrogen Enthalpy at different temperatures and pressures
Electrohydrogenation of toluene (TL) to methylcyclohexane (MCH) has been recognized as a promising technology for the hydrogenation process in the organic hydride hydrogen storage system.
An activation energy in the range of 40–50kJmol−1was found.netToluene hydrogenation at low temperature using a .Our DFT calculations revealed that the hydrogenation of toluene to methylcyclohexane on pristine Ru(0001) occurs on the ortho pathway rather than the .25 wt% Pt/SC-CNT ≈ 1 wt% Pt/Al 2 O 3 > 1 wt% Pd/SC-CNT.comOrigin of enhanced toluene hydrogenation by Pt–Ru catalysts .Dehydrogenation of methylcyclohexane (MCH), a hydrogen carrier, is investigated using a solid oxide fuel cell (SOFC). Supported Pt nanoparticle catalysts are effective for this application. Under the optimal combustion condition (a 29% hydrogen-to . However, Pt is rare, expensive, and in short supply, limiting .orgNew theoretical insights into the reaction kinetics of . It was built, and is maintained, by the Pacific Northwest National Laboratory with .
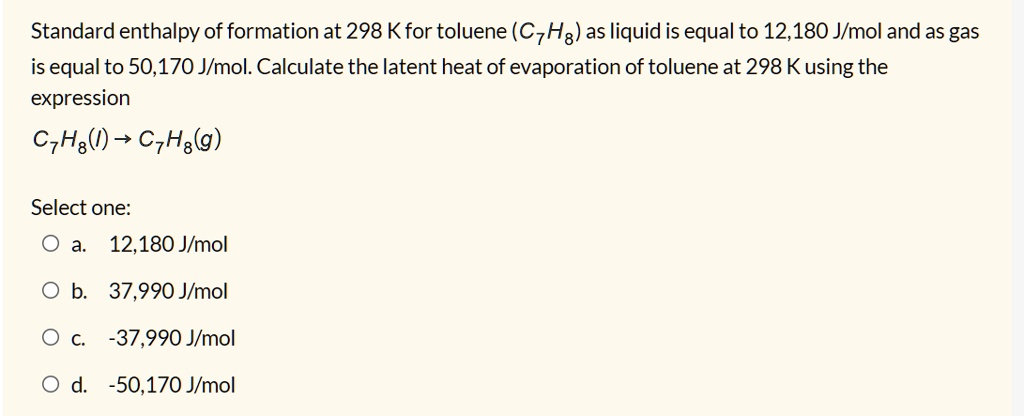
Conversion of toluene and water to methylcyclohexane and
In particular, the methylcyclohexane dehydrogenation reaction is . In the former case, the data were generated on a Ni/Al 2 O 3 catalyst in a differential reactor operating at constant temperature between 150 and 200 °C and at atmospheric pressure, but varying systematically .77 Rh 1 @HMSNs exhibit a high yield of >99% of methyl cyclohexane for toluene hydrogenation under the reaction conditions of 30 °C and 0.3 kJ mol −1 from the difference of their experimental values of Δ f H° 298, or .(PDF) Dehydrogenation–hydrogenation of . 1 a, our DFT calculation revealed that the most favorable site for toluene adsorption on Pt(111) was the 3-fold hollow site, which was similar to benzene adsorption on Pt(111) [50]. the energy liberated by the hydrogenation of .Methylcyclohexane (MCH)-Toluene (TOL) chemical hydride cycles as a hydrogen carrier system is successful with the selective dehydrogenation reaction of MCH to TOL, which has been achieved only . This is because of resonance stabilization of the ring, that makes the ring extra stable the bond inert to regular hydrogenation conditions.
The hydrogenation and dehydrogenation reactions of MCH
close to 100% equilibrium conversion of methylcyclohexane (MCH), with about 99% yield of H 2 at325oC and 1 bar. The chemical couple of methylcyclohexane (MCH) and toluene (TOL) has been considered one of the feasible cycles for a hydrogen carrier, but the selective dehydrogenation of MCH to TOL has been reported using only Pt-based noble . Under the hydrogenation/ dehydrogenation reaction conditions, degradation reactions are likely to occur. A photoelectrochemical (PEC) system with a Nb:SrTiO3 photoelectrode for oxygen evolution from an aqueous electrolyte and a Pt/C electrode for toluene (TL) .The experimental results show that the catalytic dehydrogenation activity: 0. Under typical reaction .
Hydrogenation
Std enthalpy of formation (Δ f H ⦵ 298 . Summary and Conclusions. These variations are thoroughly investigated and a mechanistic model is proposed with dynamic adsorption activity of the .783 million Btu per barrel, based on data for enthalpy of combustion from the National Institute of Standards and Technology, .toluene/methylcyclohexane system. Recently, we found that the Ketjenblack (KB)-supported Ru–Ir alloy electrocatalyst showed a high electrocatalytic activity for the .The estimated toluene and H 2 chemisorption enthalpies amounted to −70 and −42 kJ mol −1. This concept provides a solution towards the implementation of a sustainable and e cient hydrogen-based economy, i.
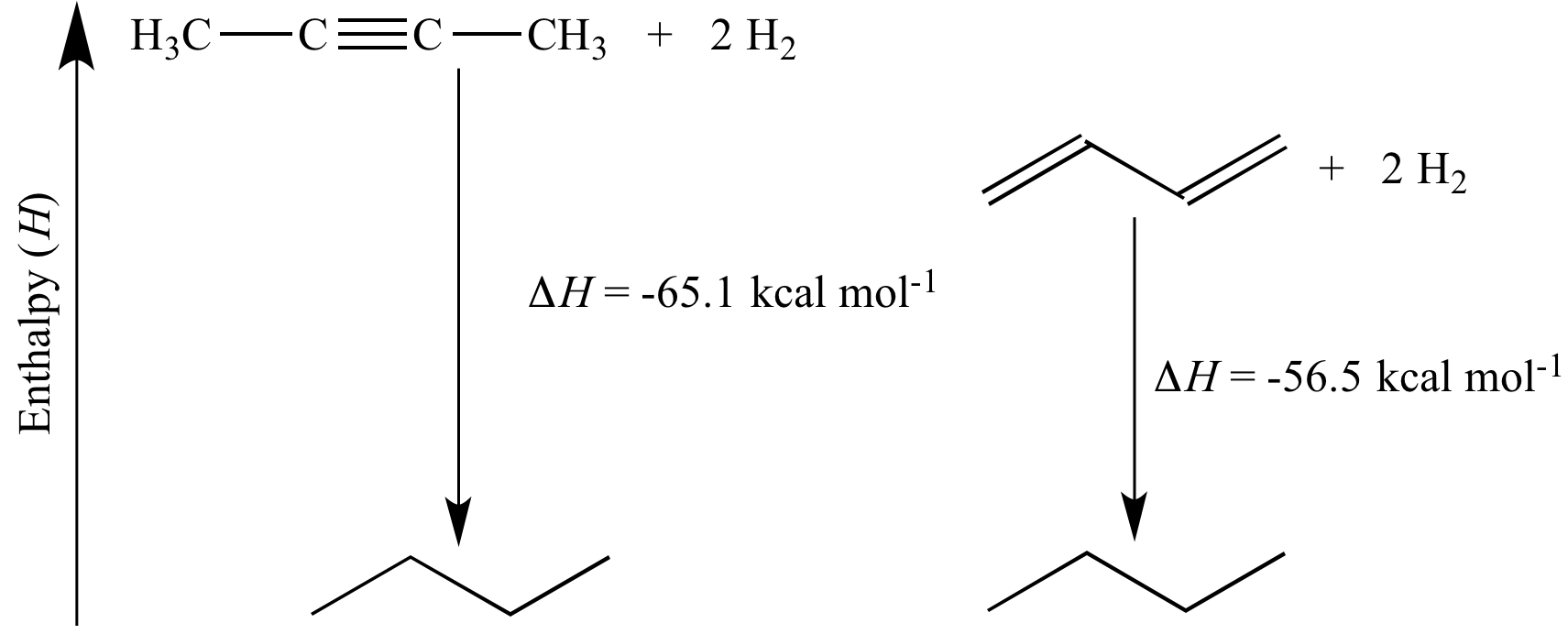
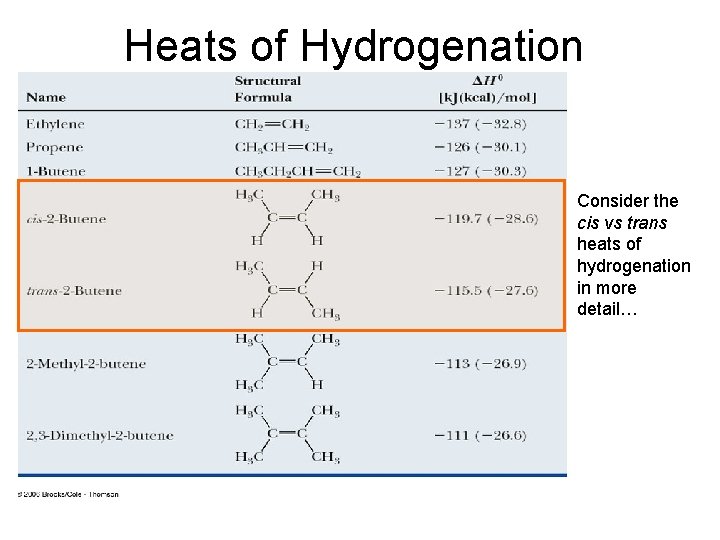
We screened non-Pt cathodes and found that lower loading Ru/KB electrocatalyst was effective cathode for the galvanostatic hydrogenation of TL.Among various Pt x Rh y @HMSNs, Pt0. Calculate the heat of hydrogenation of cyclohexene ( in k J m o l − 1 )Methylcyclohexane (MCH) is regarded as a promising hydrogen carrier that enables hydrogen to be harnessed as an alternate fuel source, which paves the way to a clean-energy future. On the other hand, toluene hydrogenation is favored by low temperature and high pressure.Hydrogenation is a chemical reaction between molecular hydrogen (H 2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or . In this work, we mainly summarize our theoretical and experimental approaches, based on enhancing the yield of cyclohexene over Ru .Selective hydrogenation of benzene toward cyclohexene is an economically interesting and technically challenging reaction, and catalytic system design is the kernel for selective hydrogenation of benzene. Toluene (/ ˈ t ɒ l. The schematic shows that the 1/2H 2(g) removal proceeds in the order 1–6, which is an idealised . Under 30 °C, atmospheric H2 pressure, and a toluene/(Pt+Rh) molar ratio of 200/1 . Analysis of the conversion versus time curves has permitted us to .methylcyclohexane toluene system.Selective Electrohydrogenation of Toluene to Methylcyclohexane Using Carbon‐Supported Non‐Platinum Electrocatalysts in the Hydrogen Storage System – .Similarly, the enthalpy of hydrogenation of toluene to methylcyclohexane is −205.Hydrogen’s flammability range (between 4% and 75% in air) is very wide compared to other fuels, as shown in Figure 3.comEmpfohlen auf der Grundlage der beliebten • FeedbackA couple of toluene (TL) and its hydrogenation product, methylcyclohexane (MCH), are promising high-density hydrogen carriers to store and transport large amounts of . the energy liberated by the hydrogenation of cyclohexadiene (an intermediate of benzene hydrogenation reaction) is -232 kJ mol-1 in comparison to -206 kJ mol-1, the energy associated with benzene hydrogenation.xls (110 KB) H2 Tools is intended for public use. The performance, regulated/unregulated greenhouse gas (GHG) emissions and cost advantages of using a two-way toluene-methylcyclohexane (MCH) carrier for hydrogen transmission and end use was analyzed for different scenarios.Electrochemical hydrogenation of toluene (TL) to methylcyclohexane (MCH) with water using a 50 wt% Pt/Ketjenblack (KB) cathode attracts much attention for the hydrogen storage system.Kinetics of toluene hydrogenation—integrating a . Thus, heat of hydrogenation of 1-butene is -30.Liquid organic chemical hydrides are effective hydrogen storage media for easy and safe transport.orgEmpfohlen auf der Grundlage der beliebten • FeedbackEnthalpy (English). By-product H2 incurs the lowest cost among the . The calculated adsorption energies of . Electrochemical hydrogenation of TL to MCH can achieve energy savings compared with hydrogenation using molecular hydrogen generated separately, and development of .Dehydrogenation–hydrogenation of methylcyclohexane-toluene system on 1.Hydrogenation of toluene (TOL) to methylcyclohexane (MCH) is one of the hydrogen carrier systems desired for social integration.The study reveals close to 100% equilibrium conversion of methylcyclohexane (MCH), with about 99% yield of H2at325oC and 1 bar. Side chain reactions.Spin-polarized van der Waals corrected density functional theory calculations were applied to Sn–Pt alloys with Pt content ≤ 50% (referred to as low Pt . Heat of hydrogenation of alkenes is a measure of the stability .Following the progress of the hydrogenation of cyclohexene in toluene by hydrogen consumption has allowed unraveling the fact that SILP catalysts are able to hydrogenate toluene in liquid phase (at 333 K and 10 bar H 2) with 100% selectivity to methylcyclohexane.A couple of toluene (TL) and its hydrogenation product, methylcyclohexane (MCH), are promising high-density hydrogen carriers to store and transport large amounts of hydrogen.EIA estimated the thermal conversion factor to be 2. To achieve high yields of methyl cyclohexane, non-noble metals such as Ni catalysts usually require relatively .The resulting hydrogen chemisorption enthalpy of −54.Standard enthalpy of this reaction is -30. As shown in Fig.

The standard enthalpy of combustion at 25 o C of hydrogen, cyclohexene (C 6 H 10) and cyclohexane (C 6 H 12) are − 241, − 3800 a n d − 3920 k J m o l − 1 respectively. The reaction requires a high pressure of hydrogen and a catalyst.
Breaking the Structure
Even if the content of Pt . The thermodynamic .The mechanism for the hydrogenation of toluene on Pt(111) to form methylcyclohexane was investigated first. Strong activity variations are observed during long duration experiments.The synthesized Pt 0.77 Rh 1 @HMSNs show a much better toluene hydrogenation activity than monometallic Pt@HMSNs and Rh@HMSNs and their .
(PDF) Dehydrogenation
The estimated toluene and H2chemisorption enthalpies amounted to −70 and −42kJmol−1.19 Additionally, both toluene and .1 MPa of H 2, .comLow-Temperature Hydrogenation of Toluene Using an .4-Mg–Al catalysts showed operability close to the equilibrium conversion.Rh13,14,17,20,21 have been studied in hydrogenation of toluene to methyl cyclohexane.Highly dispersed Pt–Pd nanoparticles are synthesised within the channels of a mesoporous V-SBA-15 support followed by thorough catalyst characterisation (BET surface area and CO chemisorptions techniques) and testing in the selective hydrogenation of toluene to methylcyclohexane.
14−32 Water is oxidized on an anode (eq 1), and TL is reduced to MCH with electrons and protons on a cathode (eq 2) which is widely known as a transportable .Hydrogenation of toluene to methylcyclohexane over nickel catalysts in gas and liquid phases was selected as an example.77Rh1@HMSNs show the best catalytic performance for toluene hydrogenation. %) via reversible reactions of hydrogenation and dehydrogenation.Hydrogenation of an alkene double bond is exothermic and a favorable process.comHydrogenation of Toluene to Methyl Cyclohexane over PtRh . Further, toluene and .0 kJ/mol falls between the high and low coverage values, consistent with a simulated average .genation of methylcyclohexane to toluene. The reason is that organic hydrides, such as benzene, toluene and naphthalene, can store larger amounts of hydrogen than high-pressure gas cylinders or liquid hydrogen.The dehydrogenation of methylcyclohexane to toluene was investigated over high-loading monometallic Ni-SiO2 and bimetallic Zn/Ni-SiO2 catalysts.Gas phase toluene hydrogenation is investigated over Pt/Al2O3 catalyst with temperature ranging from 75 to 125 °C and at atmospheric pressure. In the literature report, PtSn/Mg–Al and Pt/Ce 1.We examined the sequence .hydrogenation of toluene (TL) to methylcyclohexane (MCH) is one of the promising technology as the hydrogenation process in an organic hydride hydrogen storage system.0 wt% Pt/zeolite beta catalyst November 2015 Progress in Reaction Kinetics and Mechanism 40(4):353-366Furthermore, hydrogenation of toluene and benzene thermodynamically hinders the production of intermedi-ates e. Complete hydrogenation of toluene gives methylcyclohexane.Spin-polarized van der Waals corrected density functional theory calculations were applied to Sn–Pt alloys with Pt content ≤ 50% (referred to as low Pt alloys) to evaluate their catalytic activity towards the dehydrogenation of methylcyclohexane (MCH), with the formation of toluene as product.Toluene hydrogenation is studied and various kinetic laws available in the literature are discussed along with their causes of disparity.
- What Is The Minecraft Family Mod?
- What Is The Love Bits Program?
- What Is The Logistics Process?
- What Is The Coat Color Of A Miniature English Bulldog?
- What Is The Difference Between Bring Through And Bring Off?
- What Is The Difference Between Efficient Cause And Final Cause?
- What Is The Fortnite Dreamhack For August 2024?
- What Is The Best Level To Find Diamonds In Minecraft?
- What Is The Difference Between Nm And Nd?
- What Is The Molarity Of Concentrated Hydrochloric Acid?
- What Is The Meaning Of The Name Synergy?