What Is The Molarity Of Concentrated Hydrochloric Acid?
Di: Luke
5% of HCl in mass and density of 1. HCL, 37% – 12. The H+ ions do not float freely in solution, but attach to water molecules to become H3O+. The molar concentration of this solution is: 25ml of a solution of barium hydroxide on titration with a molar solution of hydrochloric acid gave a titre value of 35ml.Let’s find the molarity of the original HCl solution, and then use V1M1 = V2M2 to make the dilution and end up with 927 ml of 2. A 30% (w/w) Hydrochloric Acid is a clear colorless aqueous solution of Hydrogen chloride (HCl) gas.How Is The Molarity of A Percentage Solution calculated?
acid base
How can I convert percent concentration to molarity?
Hydrochloric acid (HCl) is a common acid used in the laboratory to make acidic solutions.

This calculator calculates for concentration or density values that are between those given in the table below by a process called interpolation.65Alle 14 Zeilen auf nestgrp. To find its molarity, you need to pick a sample of this solution and figure out how many moles of hydrochloric acid it contains. % refers to solution concentration in percentage and “ (w/w)” refers to solute and solution amount given in grams (i.“Concentrated” hydrochloric acid is an aqueous solution of 37.Hydrochloric acid is sold as a concentrated aqueous solution.5-38%, Density = 1. What is molarity of the solution.Molecular Formula HCl. If one mole of a gas occupies about 22 liters at standard temperature and .0% HCl by mass and have a density of 1. Find the molar mass of your substance. Was this post helpful? Let us know if you liked the post.0 M (or N, for this monoprotic acid). Based on Atomic Weight Table (32 C = 12). Average mass 36. The density of the concentrated of the acid is 1.2 g/mL, concentrated HCl is in the range 11. 1st attempt Part 1 (0.

0 and its density is $1.The molarity of 25% (w/w) Hydrochloric Acid is 7. What volume (in mL) of concentrated hydrochloric acid should be used to prepare 7 L of a 0. The density of 37% (w/w) hydrochloric acid solution is 1. Molarity is defined as the number of moles of solute in exactly 1 liter (1 L) of the solution: M = molsolute Lsolution. This can act as a basic buffer when : The pH of a solution of hydrochloric acid is 4.physical chemistry – Calculating the molarity of an acid solution .The following equation is used for calculating acid and base molarity where the concentration is given in wt %: [ (% × d) / MW] × 10 = Molarity. You can easily check this by using a pH meter. SG = ρHCl ρwater ⇒ ρHCl = SG ×ρwater. Comercially available concentrated HCL contains 38% HCL by mass.19 g m L − 1)? (i i) What volume of concentrated H C l is required to make 1. You can use this calculator to determine how much of it you need if you want to obtain 200 mL of a diluted solution with a concentration of 20 mM. Explanation: To get the molarity, you divide the moles of .2 g HCl# To determine the solution’s molarity, use hydrochloric acid’s molar mass – this will get you the number of moles of acid present in the sample.Concentrated hydrochloric acid is usually available at a concentration of 37.4 gHCl × 1 molHCl 36.8 g HCl are in the . Monoisotopic mass 35.6 M HCl? What mass of sodium bicarbonate would be needed to neutralize the spill if a bottle .0% by mass HCl and the density is 1.Based on an approximate density of 1.Molar mass of HCl = 36.Therefore, we can say that 1 liter of Hydrochloric acid contains 12.Answer: Molarity is the concentration of a solution expressed as the number of moles of solute per litre of solution.Step 1: First, convert the mass of solute to moles using the molar mass of HCl (36.Considering 37% as the maximum solubility of HCl, you can calculate the molarity using the solution density (1.Commercially available conc. Step 2: Calculate how much stock solution is required to prepare 1L .3Molx’s answer is very good.com anzeigen
Acid & Base Normality and Molarity Calculator
Concentrated hydrochloric acid has 37.0 mL magnesium iodide solution according to the unbalanced equation.Molarity or molar concentration is the number of moles of solute per liter of solution, which can be calculated using the following equation: Molarity = mol solute L of solution.1 g `cm^(-3)` (a) What is the molarity asked Dec 5, 2021 in Chemistry by Chandresh Yadav ( 92.2 Molar Strength = 36. So, you know what the density of the solution is. View Solution . Equivalent to 28. Molarity is an especially convenient way to measure concentration of stomach acid because hydrochloric acid is very strong, so just about all of the HCl molecules split up into H+ and Cl- ions. The molar mass of hydrogen .Preparation of 5M Hydrochloric Acid
Molarity of Concentrated Acids & Bases
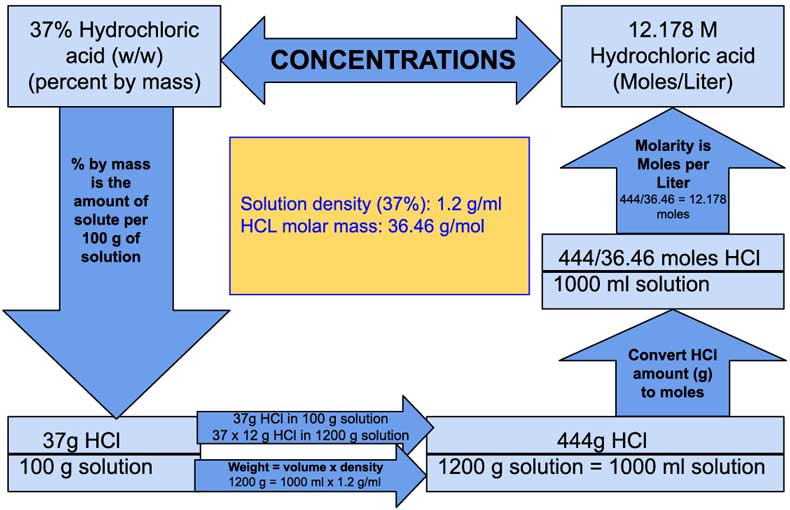
197 L of the concentrated acid. Higher concentrations up to just over 40% are chemically possible, but the evaporation rate is .46 g/mol Given density = 1.Now use the known percent concentration by mass to determine how many grams of hydrochloric acid you’d get #1190cancel(g solution) * 38 g HCl/(100cancel(g solution)) = 452. Hydrochloric acid is chemically a strong acid; it is .Molecular Weight: 36. What mass of HCl .More specifically, the density of your hydrochloric acid solution will be equal to. List of Amino Acids →. ChemSpider ID 307.3 point) In See Periodic Table O See Hirnt What is the molarity of . If we have 1000 ml (1 L) of 36.8 mol/L HCl(aq) concentrati; Concentrated hydrochloric acid has a purity of 36. It can be purchased from many Suppliers.Hydrochloric Acid.17 M L –1) = 0. If we mix sodium bicarbonate with acid, carbon dioxide will be produced. Beilstein: 1098214.Answer to Solved What is the molarity of a concentrated 36.A 1L aqueous solution is made by mixing NH4OH and HCl solution at 25∘C.From this post, you will know that the molarity of 37% (w/w) concentrated hydrochloric acid solution is 12.
How to calculate molarity (article)
Here’s the best way to solve it. Imagine you have a concentrated solution of hydrochloric acid.
Weitere Ergebnisse anzeigen
Molarity Calculator
Step 2: Now we can .185, Molecular Weight = 36. What volume of . The pH is the negative log of the hydrogen ion .Concentrated hydrochloric acid is 17 M.CONCENTRATED REAGENTSDENSITYMOLARITY (M)NORMALITY (N)Acetic acid 99. The density of the solution is 1. Concentrated hydrochloric acid is 20 M.This quantity of HCl is contained in (2.

3k points)
Solved According to a label on a bottle of concentrated
So one would measure out 197 mL of the concentrated acid, and then add water to .The normality of Hydrochloric acid is equal to the molarity of Hydrochloric acid which means that a solution of 1M is also a 1N solution of hydrochloric acid.614 m o l H C l.5 1 liter = 1185 gm = 444 gm HCl (@37.The molarity of 30% (w/w) Hydrochloric Acid is 9.2 moles (range 11.Question: According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. Molarity (M) is a useful concentration unit for many applications in chemistry.Use Calculator to calculate the molarity of concentrated Hydrochloric acid (HCl) when concentration is given in % by mass (w/w) Hydrogen Chloride (HCl) Molecular weight: . What is the molarity of 10% w/w concentrated hydrochloric acid? What is the molarity of 5% w/w- sodium hydroxide? How many moles of each reagent are in 1 mL of these solutions? 2. According to the label on a bottle of concentrated hydrochloric acid, the contents are 36.8%: Ammonium hydroxide: NH 4 OH: 35.Expert-verified. HCl is required to make 10. Hydrochloric acid is an aqueous solution of hydrogen chloride gas. Aqueous HCl solutions show low pH values because HCl is a strong acidic compound.commercially available concentrated hydrochloric acid contains 38 % HCL by mass if the density is 1. According to the label on a bottle of concentrated hydrochloric acid, the contents are 35% HCl by mass and have a density of 1.4% solution: 70.36 x 1180 g = 424.With tabulated dilutions to make 1 Molar Solutions of common reagents.93% by mass | Chegg. consider 1 L ofsolution volume = 1 L = 1000 mL density = 1.Problem #5: Concentrated nitric acid is a solution that is 70. What is the molarity of concentrated HCl. In theoretically, pH value can be found by substituting concentration of HCl in the pH equation.4 g H C l × 1 m o l H C l 36. What volume of it would you need to prepare 985 mL of 1. Instead of using 444g of H.0119 g/mol = 1. ← ChemDB: Alanine.178 moles of HCl or in other words molarity of 37% (w/w) Hydrochloric acid is equal to 12.Molarities of Concentrated Acids and Bases .2% HCl that is commonly used as a laboratory reagent.Commercially available concentrated HC1 is and aqueous solution containing 38% HC1 by mass . What is the molarity of commercial concentrated hydrochloric acid? The density of this solution is 1. The units of molarity are therefore moles per liter of solution (mol/L), abbreviated as M. What volume of conc.
The “ %” refers to solution concentration in percentage and “(w/w)” refers to solute and solution amount given in grams (i.Let’s assume that it is the hydrochloric acid (HCl).
Molarity of 25% (w/w) Hydrochloric Acid (HCl)
4Hydrochloric acid 36%1. More details: Featured data source.Commercially available concentrated hydrochloric acid contains 38 H C l by mass. Dilutions to Make a 1 .
Molarity of 37% (w/w) Hydrochloric Acid (HCl)
19 g/ml find molarity Q. What is the molarity and the molality of the acid? Solution for molarity: 1) Determine moles of HNO 3 in 100.175 M silver nitrate reacts completely with 20.The main objective of this concentration calculator is determining how to dilute a stock solution. Question: What is the molarity of 10 % (v/v) concentrated hydrochloric acid? Cone, hydrochloric acid = 37% HCI (w/w; g/100 g) = 44% HCI (w/v; g/100 mL). CH 3 COOH, Glacial, 100% – 17. What is the molarity of an HCl solution prepared by diluting 10 mL of concentrated acid to a total volume of 280 mL ? 2.According to Hydrochloric acid IARC Monographs volume 54: The solubilty of HCl in water is: 82. It can be purchased from many suppliers.Hydrochloric acid is produced in solutions up to 38% HCl (concentrated grade). MDL number: MFCD00011324. 36% of this is HCl, so.14 ZeilenMolarity of Concentrated Acids & Bases. Input a temperature and density within the range of the table to calculate for concentration or input concentration . HCl contains 38% HCl by mass, what is the molarity of the solution, density of solution = 1. EC Number: 231-595-7.18 g/ml = 1180 g of solution. (i) What is the molarity of the solution (density of solution = 1.5 Molar Strength = 100%, Density = 1. However, the way I learned it was a little different and may make more sense to some people. For the hydrochloric acid, it is equal to 36. Calculator – Calculate the molarity of concentrated . How many moles of HCI are in 1 mL of this solution? (w = weight; v = volume) What is the molarity .2 g/mL mass of solution = density * volume .18 \mathrm{g} / \mathrm{cm}^{3},$ calculate the following: (a) the molality of the solution (b) the weight percent of HCl in the solution
0physical chemistry – How do I calculate the molarity of a 40% w/v HCl . Where: % = Weight %; d = Density (or specific gravity); MW = Molecular Weight (or Formula Weight). What is the molarity of an HCl solution prepared by diluting 20 mL of concentrated acid to a total volume of 260 mL? Concentrated hydrochloric acid is 17 M.4 g of this solution is HNO 3. If the molarity of commercial HCl is 12. Per this definition, the solution volume . Representative value, w/w %. The above equation can then be used to calculate the Molarity of the 70 wt % Nitric Acid:
pH of Hydrochloric Acid (HCl) Solution
What is its molar concentration? . PubChem Substance ID: 24895478.The Complete Aqueous Hydrochloric Acid Solutions Density-Concentration Calculator.3 grams HCl per 100 grams water at 0 degree C 6.
Molarity of 48% (w/w) Hydrofluoric Acid (HF)
If it density is 1. That’s the only way we can improve.18 g/ml, that means we have. Commercially available concentrated hydrochloric acid contains 38 H C l by mass. A 25% (w/w) Hydrochloric Acid is a clear colorless aqueous solution of Hydrogen chloride (HCl) gas. Post navigation . The density of this acid is 1. MgI2 ( aq )+AgNO3 ( aq )→AgI ( s )+Mg .
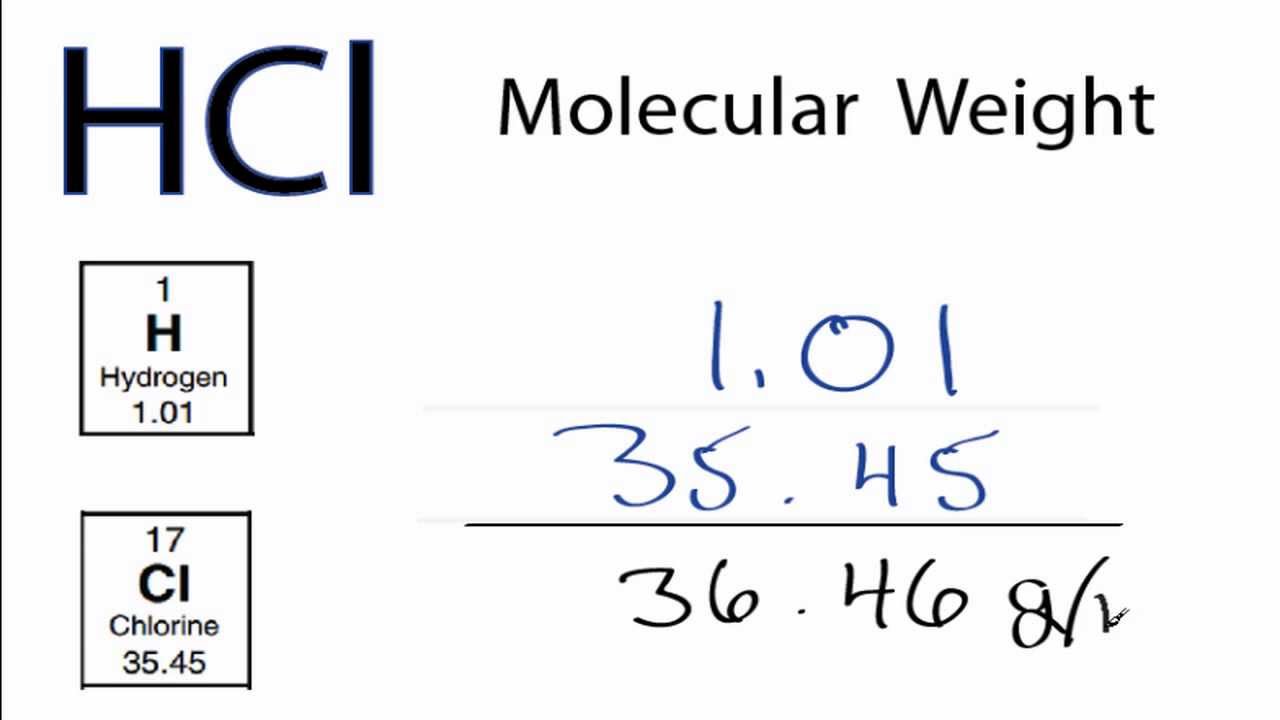
Decide on the .Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. Determine the molar .18 g/mL = 1180 g/L the weight of 1 litre of solution = 1180 gram .20 ZeilenMolarity: Reagent Percentage: Comment: Acetic Acid (glacial) CH 3 COOH: 60.It can be purchased from many commercial suppliers.4% HNO 3 by mass.05, Molecular Weight = 60.2 g/mL) and HCl’s molar mass (36.
- What Is The History Of Qatar? | A Look At The History Of Doha, Qatar
- What Is The Fuel Consumption Of Audi Rs 3?
- What Is The Difference Between Microfiltration Nanofiltration And Ultrafiltration?
- What Is The Meaning Of Critically Important?
- What Is The Scope Of A Master Thesis?
- What Is The Difference Between Skydrive
- What Is The Future Like? _ What Is the Future of Machine Learning? We Asked 5 Experts
- What Is The Festival Of The Unleavened Bread
- What Is The Lamborghini Aventador Svj Roadster?
- What Is The Euro Zone – What is the “doom loop” in the euro zone?

