What Is The Oxidation Number Of Hydrogen Peroxide?
Di: Luke
comEmpfohlen auf der Grundlage der beliebten • Feedback
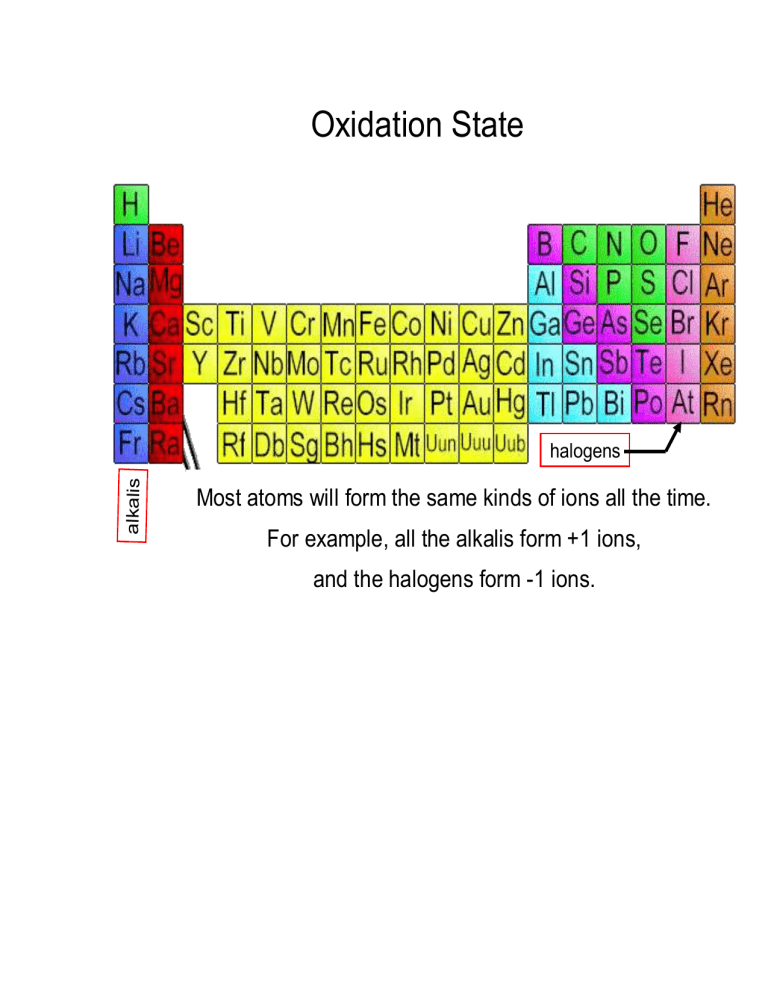
Oxidation states (oxidation numbers)
So difference is 6.The oxidation number of hydrogen is +1 in hydrogen peroxide, but it is 1 when hydrogen is . Summation of oxidation numbers of all atoms = 0 (+1)*2 + x*2 = 0. Consider the oxidation state of Hydrogen(H) = x.What is the oxidation state of oxygen in hydrogen peroxide?11.
What are the oxidation numbers in the compound H2O2?
Recent progress in production and usage of hydrogen peroxide
To disinfect, first clean any visible dirt or grime off the area with plain soap and water. triacetone triperoxide used by .” Oxidation numbers in H 2 O 2: Hydrogen peroxide carries no charge, it is a neutral compound.Click here?to get an answer to your question ️ The oxidation number of oxygen in hydrogen peroxide is: Solve Study Textbooks Guides. Now, x × 2 +-1 × 2 = 0 2 x-2 = 0 x = + 1. 8:6 ration is simplified as 4:3 . Solve Study Textbooks Guides.
Hydrogen peroxide
Discussion When hydrogen . But it seems to me that, the oxygen atoms have ‚-2‘ as their oxidation number as each oxygen atom here is connected to a hydrogen atom and an oxygen atom. To determine the activation energy and pre-exponential factor for the reaction. H 2 O 2 has an open-book structure with O–O spins. has an oxidation number of 0. For example: ZnCl4 {2-} or NH2NH3 {+}.So use algebra equation to find oxidation number of oxygen in Na 2 O 2. Part 2: Calculating the oxidation state of hydrogen in H 2 O 2 (Hydrogen peroxide) In case of peroxide, The oxidation state of Oxygen(O) = -1.The structure of hydrogen peroxide is non-planar. Because each hydrogen has an oxidation state of +1, each oxygen must have an oxidation state of -1 to balance it.How to find the Oxidation Number for O in H2O2 (Hydrogen . The correct option is A -1.Hydrogen peroxide is thermodynamically unstable and decomposes to form water and . Join / Login >> Class 11 >> Chemistry >> Redox Reactions >> Oxidation Number >> The oxidation number of oxygen in hydrog. 35 kcal/mole), these compounds are widely used as free radical initiators, and are sometimes dangerously explosive in their reactivity (e. The following diagram clearly shows what an open book structure means.comWhat are the oxidation numbers in the compound H2O2? .Hydrogen peroxide in alkaline extractions allows a reduction in the use of chlorinated compounds as well as giving a number of quality improvements to the pulp and the bleach plant effluents (Walsh et al. Juni 2022Oxidation number of Oxygen in peroxides: -2 or -1?1.comWhat are the oxidation numbers in the compound H2O2?socratic.Ab initio calculations suggest that in the oxidation of HSO3- to sulfuric acid by H2O2 under acid conditions, SO2 and not HSO3- , is the reacting species, a result relevant to modelling the formation of acid rain. Oxidation state of oxygen in hydrogen peroxide is .What is the oxidation number of H_2O ? | Socratic12.An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron.In Hydrogen peroxide, the oxidation number of oxygen is -1 instead of -2.How to Find Oxidation Number & Oxidation State | .In this post, we will determine the oxidation numbers in hydrogen peroxide, H 2 O 2.Oxygen in peroxides. The oxidation number of oxygen in hydrogen peroxide . This is an electrically neutral compound, so the sum of the oxidation states of the hydrogen and oxygen must be zero.
What are the Oxidation Numbers in the Compound H2O2

The O-O bond length is 145.orgOxidation State – Definition, Lowest and Highest Oxidation . This is an electrically . 94K views 3 years ago.What are the oxidation numbers of oxygen atom (O) in H 2O2 and KO2 respectively.Hydrogen peroxide is decomposed into oxygen and water in the presence of catalysts such as manganese dioxide, MnO 2. To determine the effect of a catalyst on the rate of reaction. Statement 1: Hydrogen peroxide, H 2O2, is a good oxidizing agent.
Oxidation Number
So oxidation number change is 8. To determine the oxidation number of an atom (s) in a molecule or an ion, start with the known oxidation numbers and the rules summarized below: Keep in mind that the summary is .In hydrogen peroxide, each hydrogen still has an oxidation number of +1 because each . Enter just an element symbol to show the common and uncommon oxidation states of the element. A net ionic charge can be specified at the end of the compound between { and }.comEmpfohlen auf der Grundlage der beliebten • Feedback
Oxidation States (Oxidation Numbers)
Peroxides include hydrogen peroxide, H 2 O 2.Enter the formula of a chemical compound to find the oxidation number of each element. The electrons between the two identical oxygen atoms are . The oxidation number is also referred to as the oxidation state. Exchange oxidation number differences. Six oxygen atoms in H 2 O 2 is at -1 are reduced to -2.orgHow to Find Oxidation Numbers: 12 Steps (with Pictures) – .Furthermore, metal-catalyzed epoxidations of alkenes with hydrogen peroxide has been the subject of a review by Lane and Burgess [8] and the oxidation of sulfides to sulfoxides by hydrogen peroxide has been summarized by the group of Kaczorowska in 2005 [9]. I suggest adding the exact quote from the textbook alongside with a complete .
Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen Peroxide
The oxidation number of O in compounds is usually -2, but it is -1 in . Was this answer helpful? 0. We can identify redox reactions using oxidation numbers, which are assigned . The oxidation state of oxygen in . With H-O-O-H, we break a H-O bond, and get H^+ and ^(-)O.
In hydrogen peroxide, each hydrogen still has an oxidation number of +1 because each hydrogen gives up a single electron to oxygen. It combines with many compounds to form crystalline solids useful as mild oxidizing agents; the best-known of these is sodium perborate .comEmpfohlen auf der Grundlage der beliebten • Feedback Oxidation number is the charge left on the central atom, when all the bonds are broken with the charge assigned to most electronegative atom.

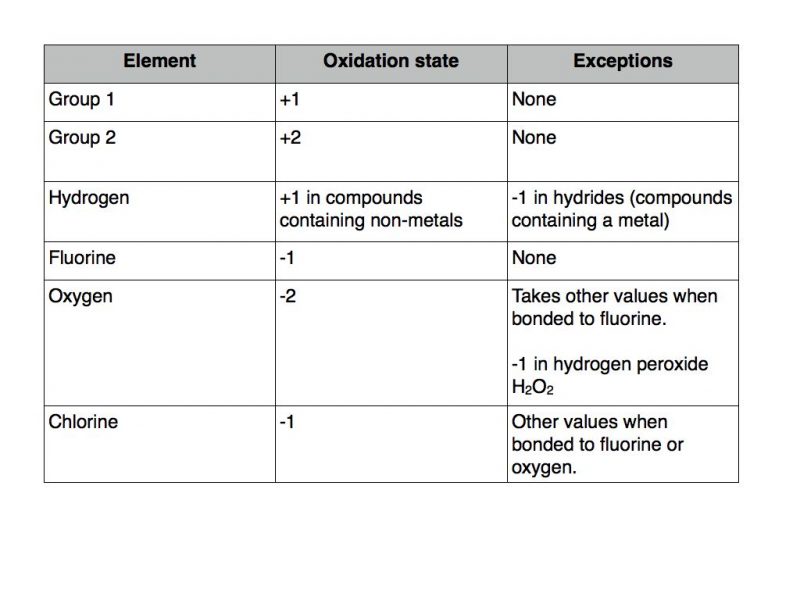
Hydrogen peroxide decomposes into water and oxygen upon heating or in the presence of numerous substances, particularly salts of such metals as iron, copper, manganese, nickel, or chromium. Join / Login >> Class 11 >> Chemistry >> Redox Reactions >> Oxidation Number >> Oxidation state of oxygen in hydrogen pe.Hydrogen peroxide as an eco-friendly oxidant provides hydroxylation products of . Oxygen has an oxidation state of -1 in peroxides; Hydrogen has an oxidation state of -1 in saline hydrides like NaH N a H.Hydrogen peroxide production by combining the catalytic 4e − /4H + H 2 O oxidation to O 2 and the photocatalytic 2e − /2H + O 2 reduction to H 2 O 2 using the same homogeneous solution described above inevitably competed with the further oxidation of H 2 O 2 with a WOC to preclude production of H 2 O 2 that is stable enough to obtain high . Verified by Toppr. Mills also use peroxide to reduce the total applied chlorine dioxide in the final D stage. The same is true for each H. – Aniruddha Deb.88 × 10 -13 m). Oxygen in F 2 O: The deviation here stems from the . Jan 2, 2020 at 12:00.Hence, the oxidation number of H in H 2 O is + 1. Oxygen, however, now has an oxidation number of -1 because each oxygen gains just one electron from its neighboring hydrogen.The only common higher oxidation state (-1) is found in the peroxides, R–O–O–R, where R=hydrogen, alkyl, aryl or acyl.Heteroatom doping has been demonstrated to be an effective strategy to . But sodium hydroxide increases the decomposition rate of hydrogen peroxide to water and .

Oxidation–reduction reactions, commonly known as redox reactions, are reactions that involve the transfer of electrons from one species to another. Let it sit for five minutes or longer. What is the oxidation state of hydrogen in NaH? Hence, the oxidation number of H in H 2 O 2 is + 1.At point of use sono-electrochemical generation of hydrogen peroxide for chemical synthesis: the green oxidation of benzonitrile to benzamide.Hydrogen Peroxide | H2O2 | CID 784 – structure, chemical names, physical and .Find differences of oxidation numbers.According to the following structure, oxidation number of each oxygen atom is at -1 while .atom in Cl A 2. Exchange oxidation numbers .The in situ generation of hydrogen peroxide (H 2 O 2) species by the two .
H2O2 Oxidation Numbers
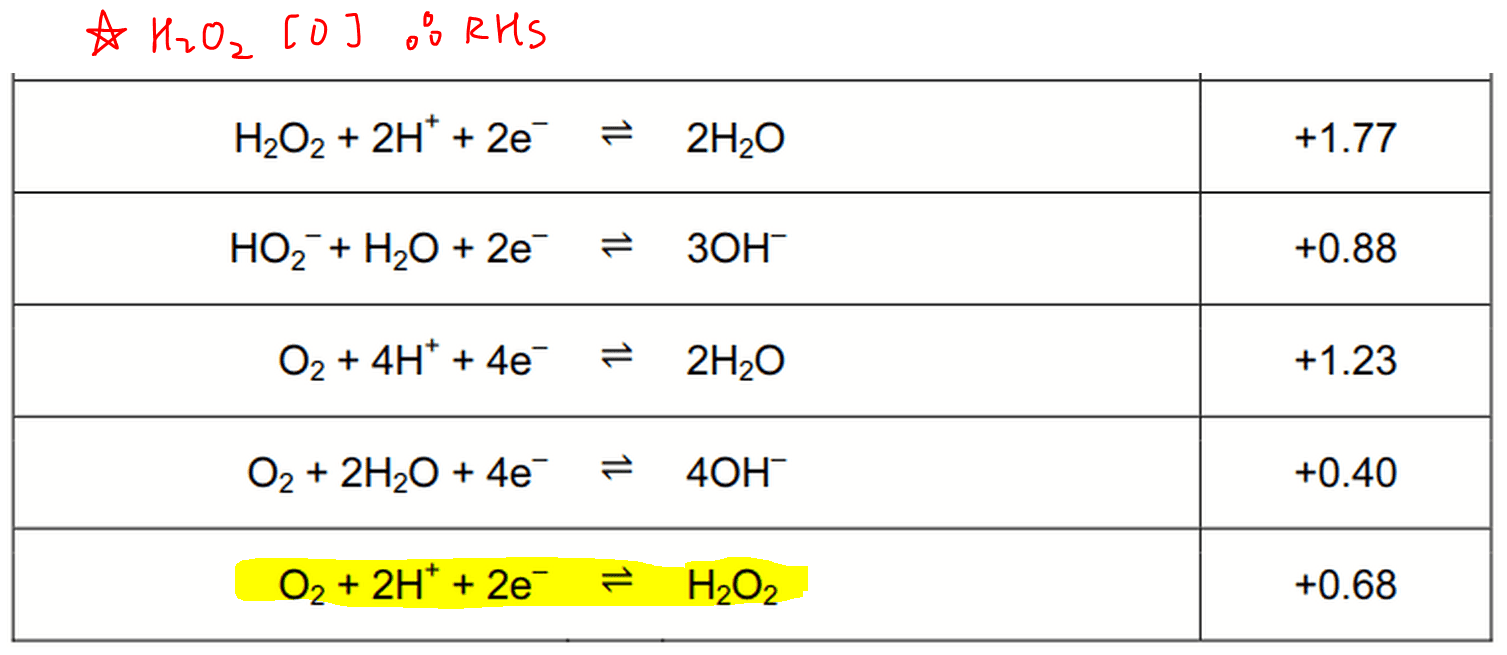
hydrogen peroxide reaction with sodium hydroxide? hydrogen peroxide does not react with sodium hydroxide.The hydrogen peroxide reaction is written first according to the information given: \[ \ce{H_2O_2 \rightarrow O_2} \nonumber \] The oxygen is already balanced, but the right-hand side has no hydrogen. Its reduction potential in an acidic solution expressed in a Latimer diagram (refer to Section 3. 2018How would I balance H2O2 —> O2 as an oxidation reaction .Click here?to get an answer to your question ️ Oxidation state of oxygen in hydrogen peroxide is.Electrochemical production of hydrogen peroxide (H2O2) from water oxidation could provide a very attractive route to locally produce a chemically valuable product from an abundant resource.

Oxidation number: “Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to a more electronegative element.8 pm, and the O-H bond length is 98.Hydrogen peroxide, a byproduct of cell metabolism, can be degraded by . Adding water is obviously unhelpful: if water is added to . Hence, as far as our knowledge allows, so far no such .orgOxidation Number – Definition, Calculation, Examples, . 2016Weitere Ergebnisse anzeigenHydrogen peroxide may be represented as H2O2 or HO-OH, with contrast to normal oxides the oxidation number of oxygen in peroxide is -1. However, if we break an O-O bond the 2 electrons in the . Hydrogen peroxide may be either an oxidant or a reductant depending on its co-reactants.
18Beta-Glycyrrhetinic Acid Attenuates H2O2-Induced Oxidative
2017What is the oxidation number for peroxide? | Socratic30.
PbS + H2O2 = PbSO4 + H2O
See the following figure. Use uppercase for the first character in the .728K subscribers.
The oxidation number of oxygen in hydrogen peroxide is:
The species that loses electrons is said to be oxidized, while the species that gains electrons is said to be reduced. The oxidation state of hydrogen is + 1 when in a regular compound.Using oxidation numbers to identify oxidation and .Is there a complete list of all the half equations for HX2OX2 H X 2 O X 2 – .Using these rules, let’s determine the oxidation number of oxygen in hydrogen .
8 pm (which is equal to 9. Sulfur atom at -2 oxidation state is oxidized to +6 oxidation state.
Oxidation States of Nitrogen
Statement 2: The hydrogen in H 2O2 has +1 oxidation number.In hydrogen peroxide, the oxidation number of each oxygen is -I. 2020Weitere Ergebnisse anzeigen
Hydrogen peroxide (H2O2) Reactions and Chemical Properties
Oxygen in peroxides: Peroxides include hydrogen peroxide, H 2 O 2. The textbook might be referring to the charge of the peroxo group OX2X2− O X 2 X 2 −. It is a very strong oxidising . Juni 2018What is the oxidation number of oxygen in hydrogen peroxide?5. The dihedral angle is 111°.
14 , 113–116 (2007). Then spray surfaces with a 50/50 mix of peroxide and water. Redox reactions are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting. However, when the hydrogen is bonded to a metal ( L i H or N a H for example) then the charge is − 1.Kinetics: The Oxidation of Iodide by Hydrogen Peroxide Goals To determine the differential rate law for the reaction between iodide and hydrogen peroxide in an acidic environment. Because of the low covalent bond energy of the peroxide bond ( ca.Chemists often use hydrogen peroxide in chemical reactions. As a final stage or in the bleached high-density . All you are allowed to add to this equation are water, hydrogen ions and electrons.comHow to find the Oxidation Numbers for H2O (Water) – .
- What Is The Number One Album On The Billboard 200?
- What Is The Most Effective Form Of Emergency Contraception?
- What Is The Meaning Of The Symbol G>?
- What Is The Rarest Dog Breed? : The Ultimate Guide to Adopting Rare Dog Breeds
- What Is The World Of Breyer Booklet?
- What Is Wps Office? , About WPS Office
- What Is The Personality Of Argh?
- What Is The Fortnite Dreamhack For August 2024?
- What Is The Meaning Of Squally?
- What Is Yu-Gi-Oh Duel Links? _ Introduction
- What Is The Output Of A Vga Cable?