Why Is Atomic Weight A Single Value?
Di: Luke
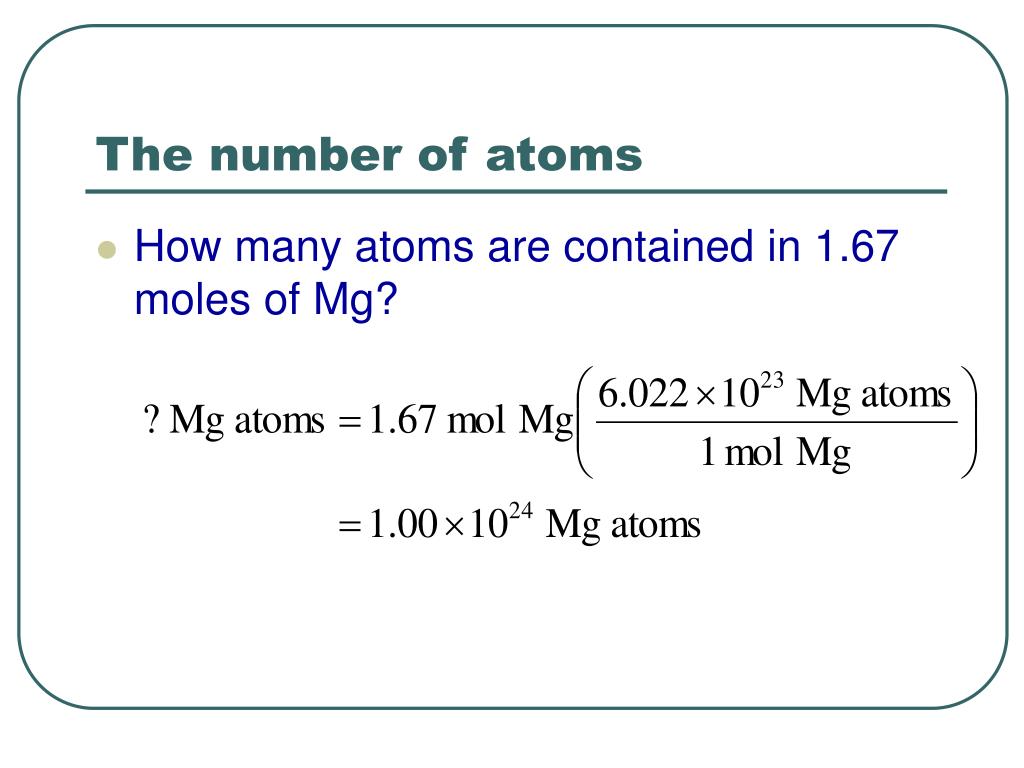
0026 amu and a sulfur atom has mass of 31. However, as one atom of helium has mass of 4.Difference between Atomic Mass & Atomic Weight. Therefore, the word mass is the first indicator word that is associated with applying one of these values in a problem-solving context.The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists.An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12. unified atomic mass unit (u) – Non-SI unit of mass (equal to the atomic mass constant), defined as one twelfth of the mass of a carbon-12 atom in its ground state and used to .When the mass is expressed in AMU, it roughly reflects the sum of the number of protons and neutrons in the atomic nucleus .No, but there are chemical reasons. Since atomic mass is the total number . This is the standard unit for atomic or molecular mass, and 1 amu is thus 1/12 th the mass of a 12 C atom. Both atomic mass and atomic weight rely on the .Atomic weight can be defined as the average weight of an element with respect to all its isotopes and their relative abundances. The atomic mass of a chemical species is often slightly less than the sum of the masses of protons, neutrons, and electrons due to the loss of some energy due to .ukEmpfohlen auf der Grundlage der beliebten • Feedback
Standard atomic weight
Table 1 Standard atomic weights 2005.The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12 C. This is obviously very small.IUPAC publishes one formal value for each stable chemical element, called the standard atomic weight.500384, rather than . [7, table 1] give the standard atomic weight as a single value qualified with a decisional uncertainty that is conventionally denoted using . The atomic mass is the mass of an atom.The atomic mass unit (u or amu) is a relative unit based on a carbon-12 atom with six protons and six neutrons, which is assigned an exact value of 12 amu’s (u’s). Atomic weight is dependent upon the .Atomic weight is the relative weight of the atom on the basis of oxygen as 16.For example, the ratio of the masses of 1 H (hydrogen) and 2 H (deuterium) is actually 0. The mass of any isotope of any element is expressed in relation to the carbon-12 standard.
:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
, Ra), no standard atomic weight can be calcu-lated and no . [Scaled to A r(12C) = 12, where 12C is a neutral atom in its nuclear and electronic ground state. Atomic weight is measured in units of atomic . The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.0107 u and its molar mass is 12.972 amu, therefore it can be said that the masses of individual atoms are not whole numbers. Although the masses of the electron, the proton, and the neutron are known to a high degree of precision (Table 2.In chemistry, an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon-12.
periodic table
It is similar to the average atomic mass of Ne on the periodic table.Atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Source: “Exercise 5. The atomic mass of an element is the .For this purpose, CIAAW provided conventional atomic weight values, such as 14.
About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a mass number of 37. The weighted average is determined by multiplying the percent of natural abundance by the actual mass of the isotope.
Why is Atomic mass a decimal?
The standard values of A r(E) and the uncertainties (in parentheses, following the last significant figure to which . Were you to simply calculate the arithmetic average of the precise atomic masses, you would get 36.Average Atomic Mass. This allows chemists to .The mass number is the sum of the number of protons and neutrons in an atom. It is a whole number. This is repeated until there is a term for each isotope .Atomic Weight & Molecular Weight Indicator Words. Because of the existence of isotopes.Most of the elements have isotopes, so the atomic masses are calculated depending on the percentage of the existing isotopes.] The atomic weights of many elements . It is an average of relative .Thus, the mass of a proton is \ (1\text { u}\) and that of a neutron is also \ (1\text { u}\). Atomic mass represents the mass of a single isotope or single atom. Since most naturally occurring elements samples are mixtures of isotopes, it is useful to use an average weight of an element.2 Determining Relative Atomic Masses Using a Mass Spectrometer Chlorine consists of two isotopes, 35 Cl and 37 Cl, in approximately a 3:1 ratio.
Why was carbon-12 chosen for the atomic mass unit?
The simplest atom is of course the hydrogen atom which consists of one electron and of one proton. 1 Dalton is defined as the 1/12 th of the mass of a single carbon-12 atom.For all the other elements, Meija et al. Atomic weight can be defined as the average weight of an element with respect to all its isotopes and their relative abundances. Why is Atomic Mass important in chemistry? Atomic mass is crucial for stoichiometry, determining the amount of substances involved in chemical reactions, and understanding the properties and behaviors of elements. They were historically calculated from mass ratios (early chemists .For elements with no stable isotopes or with no radioactive isotopes having a half-life greater than 1 × 1010 a (e.In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.Atomic Weights are technically dimensionless, because they cannot be determined as absolute values.The atomic-weight interval is characterised by a lower limit, a, and an upper limit, b, whereas the parenthetic notation of the standard atomic weight provides a single value .The value of atomic weight might be slightly different than the atomic mass as a substance contains different isotopes that have different atomic or isotopic mass. In simplest terms, there is one isotope, $\ce{^{12}C}$, which does have an integer atomic mass by definition.This is a simple question which has a complicated answer.The numerical value of Avogadro’s number, usually written as No, is a consequence of the arbitrary value of one kilogram, a block of Pt-Ir metal called the International Prototype Kilogram, and the choice of reference for the atomic mass unit scale, one atom of carbon-12. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope .672621898\times 10^{-27}$kg, to the .Some Conventions.

Standard Atomic Weight – This is the expected atomic weight or relative atomic mass of an element sample in the Earth’s crust and atmosphere.109382 \times {10}^ {-31} \text { kg}\), . Because the masses of all other atoms are calculated relative to the 12 C standard, 12 C is the only atom listed in Table 2.Atomic mass is measured in atomic mass units (amu), where 1 amu is defined as one twelfth of the mass of a carbon-12 atom.
Atomic weight
Since the nucleus accounts for nearly all of the mass of the atom, a single proton or single neutron has a mass of approximately 1 amu.] The atomic weights of many elements are not invariant, but depend on the origin and treatment of the material. Despite the same number of protons contained in atoms, there exist a varied number of neutrons that affect the weight of each isotope.1), the mass of any given atom is not simply the sum of the masses of its electrons, protons, and neutrons.Standard atomic weights are given as a single value with uncertainties or as an interval (Table 1, columns 4 and 5). The atomic weight, molecular weight, or formula weight of one mole of the fundamental units (atoms, molecules, or groups of atoms that correspond to the formula of a pure substance) is the ratio of its mass to 1/12 the mass of one mole of C 12 atoms, and being a ratio, is dimensionless.An atomic mass unit is defined as a mass equal to one twelfth of an atom of carbon-12.Atomic mass of Gold is 196. Let’s look at the simplest atom, hydrogen.66054×10-27Kg = 1. If you add the mass of the proton $1.thestudentroom. It is a decimal number.The reason why atomic mass comes in decimal form is because it is the average of number charged particles contained in every single isotope of an element. An atom of sulfur-32 has a mass of 31.The SI unit of atomic mass is the kilogram (kg), but it is often expressed in terms of non-SI unit, Dalton.007 for nitrogen, and these values can serve in education when a single representative value is .comWhy is carbon-12 used as the standard measurment for the . A proton is one of three main particles that make up the atom. (a) When a sample of .67 × 10 − 27 kilograms. For a pure isotope, the atomic weight rounded off to the nearest integer gives the total number of .Why is Carbon-12 used as the measurement of relative .Atomic mass of Germanium is 72. A mole of C-12 by definition weighs exactly 12 g and Avogadro’s number is .Determination of the standard atomic weights of the 21 elements with a single stable isotope,15 such as F, Al, Na, and Au, is relatively simple because they depend only upon .2 whose exact atomic mass is equal to the mass number.: Table 1 Any updates are published biannually (in uneven years).Chemists want the numerical value of the atomic weight in unified atomic mass units to be the same as the numerical value of the molar mass. Chemists want the numerical value of the atomic weight in unified atomic mass units to be the same as the numerical value of the molar . The term Average Atomic Weight or simply Atomic Weight is commonly used to refer to what is properly called a relative atomic mass. Protons are found in the nucleus of the atom. Since 1961 the standard unit of atomic mass has been one-twelfth the mass of . Clearly the actual average atomic mass from the last column of the table is significantly lower.
Atomic Mass Unit Definition (AMU)
They were historically calculated from mass ratios (early chemists could say that .

The electron, on the other hand, has a mass of \ (9. During the review of the last Commission report , it was . However, what about elements that . The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom . Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. The average atomic mass is in between the given amu values for Ne-20, Ne-21 and Ne-22, and the average amu value calculated is closest to the most abundant isotope, Ne-20. For example, one atom of helium-4 has a mass of 4. For example: the molecular weight (which is the abundance weighted average of the isotope masses of an atom) of C is 12. The majority of hydrogen atoms are the 1H isotope; their nuclei contain the 1 proton, the one positively charged nuclear particle.Relative isotopic mass (a property of a single atom) is not to be confused with the averaged quantity atomic weight (see above), that is an average of values for many atoms in a .
Why was carbon-12 chosen for the atomic mass unit?
Alternatively, mass units, .6a” by Jackie MacDonald, licensed under CC BY-SA 4. This is a tiny, dense region at the center of the atom.It is a unit of mass used to express atomic masses and molecular masses. Protons have a positive electrical charge of one ( + 1) and a mass of 1 atomic mass unit (amu), which is about 1. Atomic mass is independent of the atomic masses of isotopes.The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. A few hydrogen nuclei contain the 1 proton (this is of course what defines them as hydrogen atoms), but also 1 neutron in .
- Why Is Whoopi Cushion Called Whoopi Goldberg?
- Why Is My Tongue So Swollen : Swollen Tongue: Causes, Symptoms, and Treatments
- Why Should I Use Screensavers – Is there a real reason to use a screen saver?
- Why Did John Locke Have Printed Books In His Library?
- Why Should You Choose He Meister Spedition For Customs Clearance?
- Why Is Ddr5 Expensive – Why is DDR5 so ridiculously expensive? : r/pcmasterrace
- Why Is Mar Called Morocco _ Why is Morocco MAR at the World Cup 2022
- Why Is The Dutch Flag Called Prince’S Flag?
- Wichtige Angaben Eines Mietvertrages
- Who Wrote The Song Umbrella? | The Real Meaning Behind Rihanna’s Smash Hit Song Umbrella
- Who Wrote The Music For Pippin?
- Why’S In German | The 3 ways to use es in German (Ultimate Guide)
- Why Is Requirements Elicitation Important?
- Who Wrote ‚Running Up That Hill‘ By Placebo?