Why Is Copper A Well-Known Element?
Di: Luke
Copper is widely known for its malleability and ductility, making it an excellent choice for many everyday items.
An adult human needs around 1. The occurrence of this metal in Earth’s crust is about 0.546 and a mass number of 63. It can be found in ores, minerals and also solid in elemental form. But there are few more things to know about this . Easily identifiable because of its iridescent, golden red color, copper and its alloys, have been used by humans for thousands of years. And, its antimicrobial property is becoming increasingly important to the . They are non-lustrous, brittle and poor conductors of heat and electricity (except graphite). Federal government websites often end in .Copper is a well-known element, and its willingness to alloy with other metals and its working and ductility properties have been known since the dawn of civilization.Luckily, copper wiring reduces the chances of these electrical “faux pas”.The chemical symbol for Boron is B.
![]()
Look up properties, history, uses, and more. These include contributions to food supply, infrastructure, CO 2 reduction and sustainable development. The copper industry works to engage employees, communities and governments in addressing the challenges encountered in providing essential materials for society .Copper is a metal and like other metals, it is also malleable.Copper is a ductile and malleable base metal that is valued for its high thermal and electrical conductivity. It is the second-best conductor of electricity, . Copper is a mineral and an element essential to our everyday lives.5 to 3 on the Mohs scale of hardness. highlight the diverse roles of copper in biology.
Copper: An Essential Resource
It has a metallic luster, and registes at a 2. The atoms of copper are arranged in face-centered cubic lattice i.Copper and the Critical Minerals List. Metallic copper is well known to undergo corrosion in air and ultimately produce a characteristic green color when converted to a copper salt such as copper .
copper alloy
An element is any chemical substance that cannot be broken down any further by ordinary chemical processes. In other cases, the metal is precious because it is valued for other uses and is rare. Non-metals can be gaseous, liquids or solids. Apart from gold, copper is the only metal that does not naturally occur in gray or silver color.Converting copper ore to copper metal often involves many steps.Copper is a transition element and has the symbol Cu and atomic number 29.Copper has long been known to be an essential element for human health, but the Berkeley team has found that copper is responsible for moving fat out of fat cells and into the blood stream for use as .
Chemistry of Copper, the Transition Metal
Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure. The earliest known objects made .
Copper: An essential metal in biology
Copper is a soft, malleable metal that conducts electricity and heats exceptionally well. Copper has been . There are over 100 different borate minerals, but the most common are: borax, . Copper ions can adopt distinct redox states oxidized Cu (II) or reduced (I), allowing the metal to play a pivotal role in cell physiology as a catalytic cofactor in the redox chemistry of enzymes, mitochondrial respiration, iron absorption, free radical . They have high resistance against corrosion.

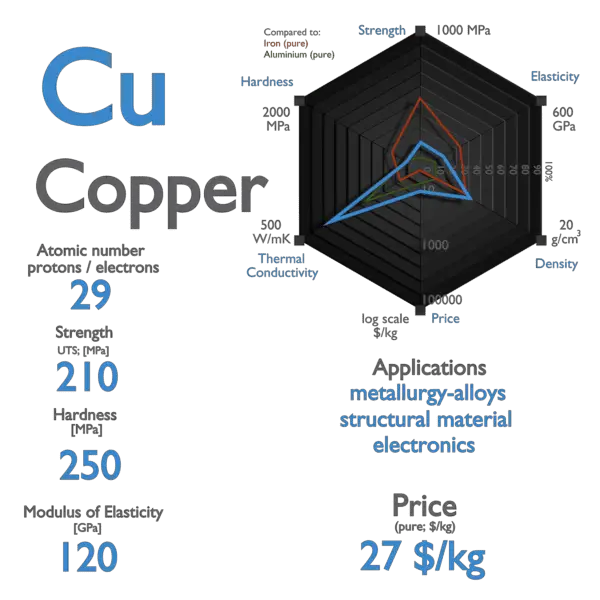
Copper is an element that is often found in the earth’s crust. Imagine being at a rock concert, and the sound system fails. It also has two valence electrons that provide a .Copper is a chemical element with an atomic number of 29 in the periodic table of elements.Atomic Number: 29.
Why Are Some Metals More Conductive than Others?
Electron Configuration : [Ar] 3d 10 4s 1.
Copper, Chemical Element
the copper atoms are located at each corner of a cube as well as at the center of the cube. It is a major industrial metal because of its high ductility, malleability, thermal and electrical conductivity and resistance to corrosion. It has an atomic weight of 63. In addition, copper is used to make . Early humans used copper for many purposes, including jewelry, tools, and weapons. Copper, which is classified as a transition metal, is solid at ambient temperature.
Copper
It is extensively mined across the .
Copper
Truro School in Cornwall.
Why Copper Is a Critical Mineral
It’s roughly the 25th most abundant chemical element in Earth’s crust and is found throughout the world, from the Andes mountains of Chile (the leading producer, which generates just under a third of the world’s copper) to the craggy Cornish coastline in .Copper is an element with the chemical symbol Cu and atomic number 29. Before sharing sensitive information, make sure you’re on a federal government site. The USGS defines a Critical Mineral as having three components, and copper meets each one: It is essential to economic and national security.2 milligrams of copper a day, to help enzymes transfer energy in cells. Excess copper is toxic. Copper has twenty-nine protons and thirty-four neutrons in its nucleus, and twenty-nine electrons in four shells. Element Symbol: Cu. Elements make up all matter in . Learn interesting geological facts about copper – where to find it, top producers, and why to invest.

However, within the last decade, a new role for redox .Autor: Anne Marie Helmenstine, Ph. Geology of Copper. Verified by Toppr. Here is how you know.It cannot be broken down into simpler substances by ordinary chemical means. Copper ions can adopt distinct redox states oxidized Cu(II) or reduced (I), allowing the metal to . It has a wide variety of applications in modern life, domestically, in industry, and biologically.Precious metals are elemental metals that have high economic value. Block: d-block.For example, pure copper (Cu) is more conductive than a copper alloy—a substance combining copper and one or more other chemical elements, each of which is . The most widely known precious metals are corrosion-resistant metals that are used in jewelry, currency, and investments. The slurry is spun around in large vats with steel balls to crush/the ore to an even finer powder. The combination of these properties leads to the wide application of copper for engineering and .
Copper biology: Current Biology
Pure copper does not corrode; over time, it interacts with air to generate a coating of grey-green . At one time, it could be found lying on the ground in its native state or uncombined state. Metals form metallic bonds, which are a thing of its own. Well, this was just a simple answer. However, copper has no unpaired electrons in .Updated on November 29, 2019. Copper is a trace element, important for the function of many cellular enzymes. So according to the definition that transition metal cations have partially filled (n-1)d subshell, copper can be regarded as a transition metal.Precious Metals Resources. Copper occupies the same family of the periodic table as silver and gold, since they each have one s-orbital electron on top of a filled electron shell . Importance in High Voltage Scenarios.Copper (I) chemistry is limited by a reaction which occurs involving simple copper (I) ions in solution.Copper is well known to serve as a static cofactor in a variety of fundamental cellular processes.Copper is an essential element. It is an essential nutrient in our daily diet. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. But despite copper’s indispensable role in the modern economy, it is not on the U. The best known traditional types are bronze, where tin is a significant addition, and brass, using zinc instead. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. It is a reddish-orange malleable metal with high thermal and electrical conductivity. Regardless of how it is gathered, however, this is where most items begin to open up as . Due to its effectiveness as an electrical conductor, copper is now most often found in related .Copper is the 29th element in the periodic table and has a symbol of Cu and atomic number of 29. However, within the last decade, a new role for redox-active copper has emerged in nutrient sensing and protein regulation. It is a reddish-colored metal, is malleable and ductile, is resistant to corrosion, and is a good conductor of heat and electricity.Chemistry, Earth. Genetic diseases, such as Wilson’s disease and Menkes’ disease, can affect the body’s ability to .Inorganic elements, such as copper (Cu), iron and zinc, once solubilized from the Earth’s crust, are neither created nor destroyed and therefore their homeostatic .
Is copper an element or molecule?
Both these are imprecise terms, both having been commonly referred to as lattens in the past. The name copper is derived from the Latin word ‘cuprum’, which means “metal of Cyprus”, an island where copper was mined during the Roman period.Copper is defined as an element in the periodic table.Copper is critical for everything from the electrical grid to electric vehicles and renewable energy technologies. Copper is used in coins, pipes, electrical wiring, electronic components, and the human body. First, the ore is crushed into small pieces.Copper is a chemical element that is also a metal. While its electrical properties, in combination with its ductility and malleability, have helped .Copper alloys are metal alloys that have copper as their principal component. Was this answer helpful?
Copper biology: Current Biology
Copper is considered a pure substance because it is an element, composed solely of copper atoms, and has a consistent and definite chemical composition throughout. Then the crushed pieces are mixed with water to form a slurry, a soup-like mixture of crushed ore and water.Overview
Copper Facts: Chemical and Physical Properties
This is due to the crystal structure of copper.Copper is a trace element, important for the function of many cellular enzymes. Copper (I) ions in solution disproportionate to give copper (II) ions and a precipitate of copper.
In some cases, the metals have been used as currency. Atomic Mass : 63. Element Family: Transition Metal. There are copper . Discovery: The first . It is said that copper has a higher melting point than zinc because of the d electrons in copper being involved in metallic bonding.gov means it’s official. This is a good example of disproportionation – a reaction in which something oxidises and reduces itself.25%, concentrated in copper ores. The reaction is:As copper is a great conductor of electricity it is used to make wires that carry electricity into homes, schools and businesses. Copper, the red metal, is one of the most electrically conductive of all the metal elements. Hence, C u + 2 has 3 d 9 configuration.Copper has an important role to play in addressing issues critical to society. It plays a key role in . It is located in group eleven, period four and block d of the periodic table. Elements that tend to gain electrons to form anions during chemical reactions are called non-metals. Also, internal planes are present in between the layers of the atoms. It is also ductile and has an interesting reddish-brown color.546 (3) Group: 11.Copper was one of the earliest elements known to man.Aside from this, copper ingots can be dropped by drowned as well. An easy way to rule out molecular bonding is if the boiling point is high; most metals . Its low resistance ensures that electricity flows smoothly, minimizing the risk of sudden interruptions or short circuits. Copper is a transition metal, one of several . An official website of the United States government.Copper is a transition metal element with the chemical symbol Cu and atomic number 29. Copper is a transition element and has the symbol Cu and atomic number 29.Copper is often found on its own as native copper, but may grow together with silver, or may show impurities. Copper is safe, reliable and long-lasting—these properties added together represent the best, most cost-effective choice over the lifetime of critical underground .
Copper: introduction to the chemical element
Although copper has 3 d 10 configuration ,it can lose one electron from this arrangement. These are electronegative elements. Its key properties are that it has excellent electrical conductivity, high thermal conductivity, and good corrosion resistance.Copper is a relatively soft, reddish metal that conducts heat and electricity well.Chemical element, Copper, information from authoritative sources. In this Primer, Tsang et al.Copper is a well-known element because of its distinctive reddish metallic color and because it occurs in pure form in daily life. It is a reddish . Copper’s distinctive red color made it easy to identify.Element Copper (Cu), Group 11, Atomic Number 29, d-block, Mass 63.The properties of copper piping, what is in it, what can leach from it, the associated potential health and safety effects, and how leaching can be controlled are quantified and well-known.
- Why Did Abraham Lincoln Challenge Stephen Douglas?
- Why Should You Consider Implementing Caffeine Substitutes?
- Why Did Jimmy Fallon Leave Snl?
- Why Are Some Hats Positioned Wrong In Team Fortress 2?
- Why Choose Eibach For Your Project?
- Who Wrote The Song Umbrella? | The Real Meaning Behind Rihanna’s Smash Hit Song Umbrella
- Why Can’T I Backup My Iphone? – Can’t backup iPhone photos to PC
- Why Do People Get Obsessed – Why Do I Have OCD?
- Why Is Tec De Monterrey Gaining A Reputation?
- Why Did We Predict The World Cup In Qatar?
- Why Is Wiener Sausage Called Wiener?
- Why Am I Interested In Marketing
- Why Is Riverdale So Popular _ 10 reasons why Riverdale is actually pretty awesome